Concurrent Anemia and Elevated C-Reactive Protein Predicts HIV Clinical Treatment Failure, Including Tuberculosis, After Antiretroviral Therapy Initiation
- PMID: 25828994
- PMCID: PMC4542913
- DOI: 10.1093/cid/civ265
Concurrent Anemia and Elevated C-Reactive Protein Predicts HIV Clinical Treatment Failure, Including Tuberculosis, After Antiretroviral Therapy Initiation
Abstract
Background: Anemia is a known risk factor for clinical failure following antiretroviral therapy (ART). Notably, anemia and inflammation are interrelated, and recent studies have associated elevated C-reactive protein (CRP), an inflammation marker, with adverse human immunodeficiency virus (HIV) treatment outcomes, yet their joint effect is not known. The objective of this study was to assess prevalence and risk factors of anemia in HIV infection and to determine whether anemia and elevated CRP jointly predict clinical failure post-ART.
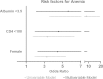
Methods: A case-cohort study (N = 470 [236 cases, 234 controls]) was nested within a multinational randomized trial of ART efficacy (Prospective Evaluation of Antiretrovirals in Resource Limited Settings [PEARLS]). Cases were incident World Health Organization stage 3, 4, or death by 96 weeks of ART treatment (clinical failure). Multivariable logistic regression was used to determine risk factors for pre-ART (baseline) anemia (females: hemoglobin <12.0 g/dL; males: hemoglobin <13.0 g/dL). Association of anemia as well as concurrent baseline anemia and inflammation (CRP ≥ 10 mg/L) with clinical failure were assessed using multivariable Cox models.
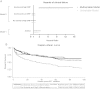
Results: Baseline anemia prevalence was 51% with 15% prevalence of concurrent anemia and inflammation. In analysis of clinical failure, multivariate-adjusted hazard ratios were 6.41 (95% confidence interval [CI], 2.82-14.57) for concurrent anemia and inflammation, 0.77 (95% CI, .37-1.58) for anemia without inflammation, and 0.45 (95% CI, .11-1.80) for inflammation without anemia compared to those without anemia and inflammation.
Conclusions: ART-naive, HIV-infected individuals with concurrent anemia and inflammation are at particularly high risk of failing treatment, and understanding the pathogenesis could lead to new interventions. Reducing inflammation and anemia will likely improve HIV disease outcomes. Alternatively, concurrent anemia and inflammation could represent individuals with occult opportunistic infections in need of additional screening.
Keywords: CRP; HIV; anemia; antiretroviral therapy; inflammation.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Moh R, Danel C, Messou E, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS 2007; 21:2483–91. - PubMed
-
- Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS 2007; 21:713–9. - PubMed
-
- Paton NI, Sangeetha S, Earnest A, Bellamy R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Med 2006; 7:323–30. - PubMed
-
- Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med 2004; 116(suppl 7A):27S–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

