Pharmacokinetic and pharmacodynamic profile of bendamustine and its metabolites
- PMID: 25829094
- PMCID: PMC4441746
- DOI: 10.1007/s00280-015-2727-6
Pharmacokinetic and pharmacodynamic profile of bendamustine and its metabolites
Abstract
Purpose: Bendamustine is a unique alkylating agent indicated for the treatment of chronic lymphocytic leukemia and rituximab-refractory, indolent B cell non-Hodgkin's lymphoma. Despite the extensive experience with bendamustine, its pharmacokinetic profile has only recently been described. This overview summarizes the pharmacokinetics, pharmacokinetic/pharmacodynamic relationships, and drug-drug interactions of bendamustine in adult and pediatric patients with hematologic malignancies.
Methods: A literature search and data on file (including a human mass balance study, pharmacokinetic population analyses in adult and pediatric patients, and modeling analyses) were evaluated for inclusion.
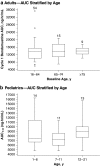
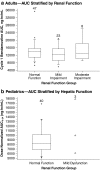
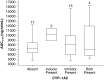
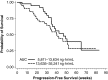
Results: Bendamustine concentrations peak at end of intravenous infusion (~1 h). Subsequent elimination is triphasic, with the intermediate t 1/2 (~40 min) as the effective t 1/2 since the final phase represents <1 % of the area under the curve. Bendamustine is rapidly hydrolyzed to monohydroxy-bendamustine and dihydroxy-bendamustine, which have little or no activity. Cytochrome P450 (CYP) 1A2 oxidation yields the active metabolites γ-hydroxybendamustine and N-desmethyl-bendamustine, at low concentrations, which contribute minimally to cytotoxicity. Minor involvement of CYP1A2 in bendamustine elimination suggests a low likelihood of drug-drug interactions with CYP1A2 inhibitors. Systemic exposure to bendamustine 120 mg/m(2) is comparable between adult and pediatric patients; age, race, and sex have been shown to have no significant effect on systemic exposure in either population. The effect of hepatic/renal impairment on bendamustine pharmacokinetics remains to be elucidated. Higher bendamustine concentrations may be associated with increased probability of nausea or infection. No clear exposure-efficacy response relationship has been observed.
Conclusions: Altogether, the findings support dosing based on body surface area for most patient populations.
Figures



References
-
- Pönisch W, Rozanski M, Goldschmidt H, et al. Combined bendamustine, prednisolone and thalidomide for refractory or relapsed multiple myeloma after autologous stem-cell transplantation or conventional chemotherapy: results of a phase I clinical trial. Br J Haematol. 2008;143:191–200. doi: 10.1111/j.1365-2141.2008.07076.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

