Pharmacological and Protein Profiling Suggests Venetoclax (ABT-199) as Optimal Partner with Ibrutinib in Chronic Lymphocytic Leukemia
- PMID: 25829398
- PMCID: PMC4537801
- DOI: 10.1158/1078-0432.CCR-14-2809
Pharmacological and Protein Profiling Suggests Venetoclax (ABT-199) as Optimal Partner with Ibrutinib in Chronic Lymphocytic Leukemia
Abstract
Purpose: Bruton's tyrosine kinase (BTK) is a critical enzyme in the B-cell receptor pathway and is inhibited by ibrutinib due to covalent binding to the kinase domain. Though ibrutinib results in impressive clinical activity in chronic lymphocytic leukemia (CLL), most patients achieve only partial remission due to residual disease. We performed a pharmacologic profiling of residual circulating CLL cells from patients receiving ibrutinib to identify optimal agents that could induce cell death of these lymphocytes.
Experimental design: Ex vivo serial samples of CLL cells from patients on ibrutinib were obtained prior and after (weeks 2, 4, and 12) the start of treatment. These cells were incubated with PI3K inhibitors (idelalisib or IPI-145), bendamustine, additional ibrutinib, or BCL-2 antagonists (ABT-737 or ABT-199), and cell death was measured. In vitro investigations complemented ex vivo studies. Immunoblots for BTK signaling pathway and antiapoptotic proteins were performed.
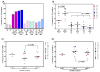
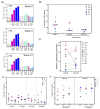
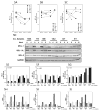
Results: The BCL-2 antagonists, especially ABT-199, induced high cell death during ex vivo incubations. In concert with the ex vivo data, in vitro combinations also resulted in high cytotoxicity. Serial samples of CLL cells obtained before and 2, 4, 12, or 36 weeks after the start of ibrutinib showed inhibition of BTK activity and sensitivity to ABTs. Among the three BCL-2 family antiapoptotic proteins that are overexpressed in CLL, levels of MCL-1 and BCL-XL were decreased after ibrutinib while ABT-199 selectively antagonizes BCL-2.
Conclusions: Our biologic and molecular results suggest that ibrutinib and ABT-199 combination should be tested clinically against CLL.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

