T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses
- PMID: 25829541
- PMCID: PMC4386370
- DOI: 10.1073/pnas.1418549112
T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses
Abstract
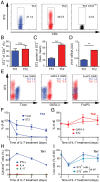
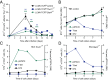
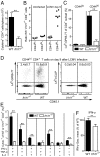
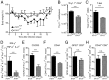
During infection, the release of damage-associated molecular patterns, so-called "alarmins," orchestrates the immune response. The alarmin IL-33 plays a role in a wide range of pathologies. Upon release, IL-33 signals through its receptor ST2, which reportedly is expressed only on CD4(+) T cells of the Th2 and regulatory subsets. Here we show that Th1 effector cells also express ST2 upon differentiation in vitro and in vivo during lymphocytic choriomeningitis virus (LCMV) infection. The expression of ST2 on Th1 cells was transient, in contrast to constitutive ST2 expression on Th2 cells, and marked highly activated effector cells. ST2 expression on virus-specific Th1 cells depended on the Th1-associated transcription factors T-bet and STAT4. ST2 deficiency resulted in a T-cell-intrinsic impairment of LCMV-specific Th1 effector responses in both mixed bone marrow-chimeric mice and adoptive cell transfer experiments. ST2-deficient virus-specific CD4(+) T cells showed impaired expansion, Th1 effector differentiation, and antiviral cytokine production. Consequently, these cells mediated little virus-induced immunopathology. Thus, IL-33 acts as a critical and direct cofactor to drive antiviral Th1 effector cell activation, with implications for vaccination strategies and immunotherapeutic approaches.
Keywords: CD4 T cell; IL-33; ST2; Th1 cell; virus infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Oppenheim JJ, Yang D. Alarmins: Chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–365. - PubMed
-
- Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7(6):321–329. - PubMed
-
- Schmitz J, et al. IL-33, an interleukin-1–like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

