Human lung myofibroblast TGFβ1-dependent Smad2/3 signalling is Ca(2+)-dependent and regulated by KCa3.1 K(+) channels
- PMID: 25829947
- PMCID: PMC4379608
- DOI: 10.1186/s13069-015-0022-0
Human lung myofibroblast TGFβ1-dependent Smad2/3 signalling is Ca(2+)-dependent and regulated by KCa3.1 K(+) channels
Abstract
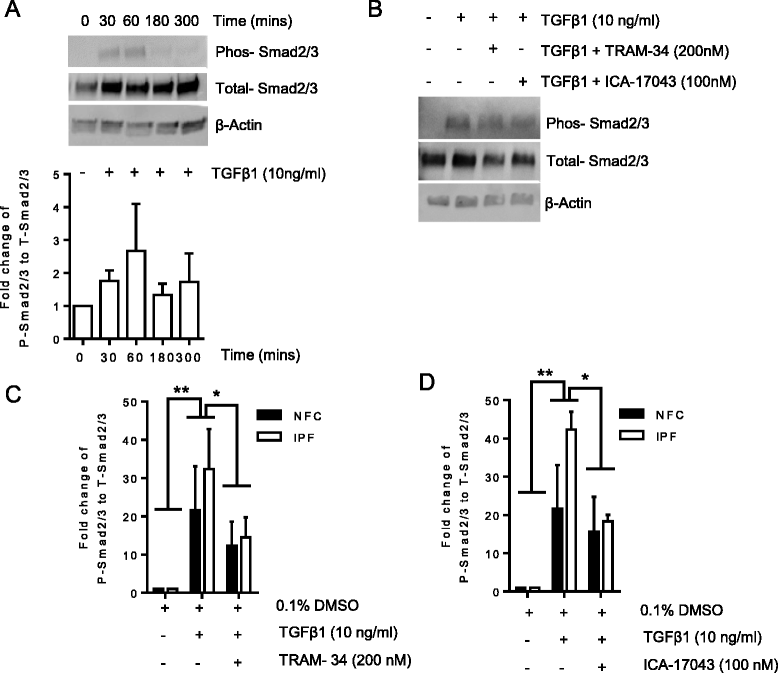
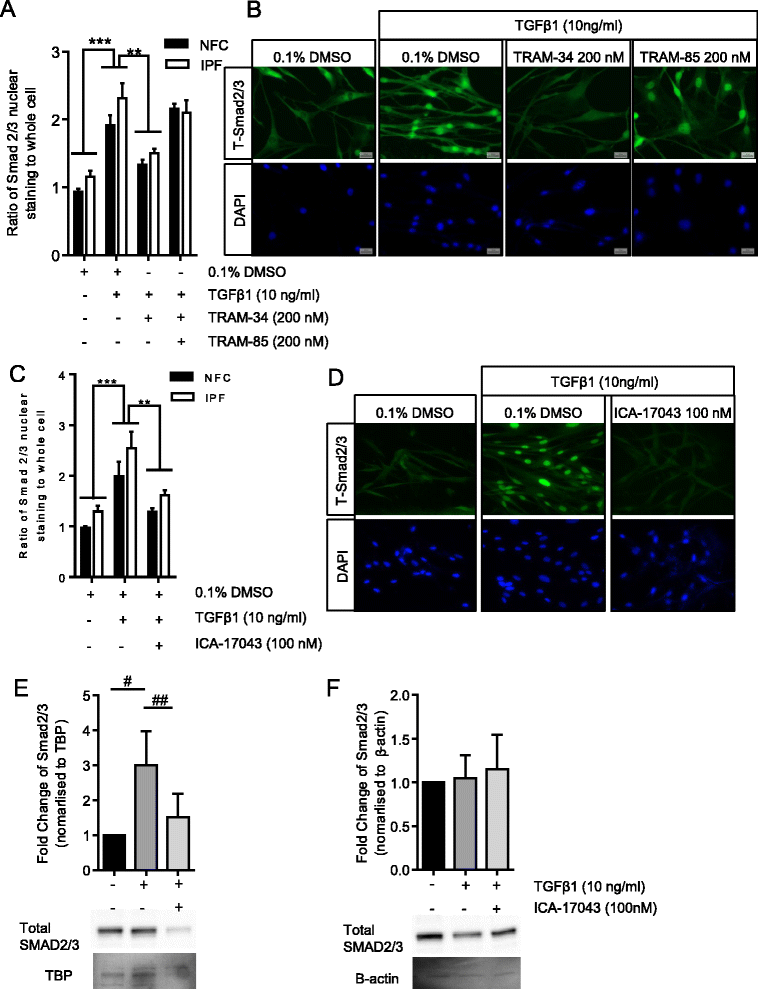
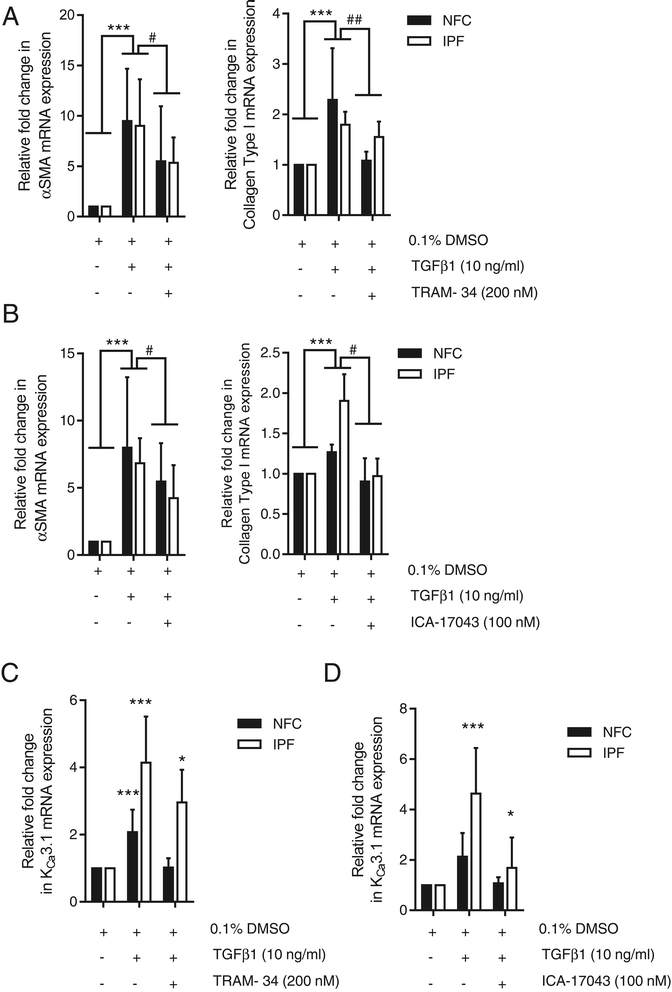
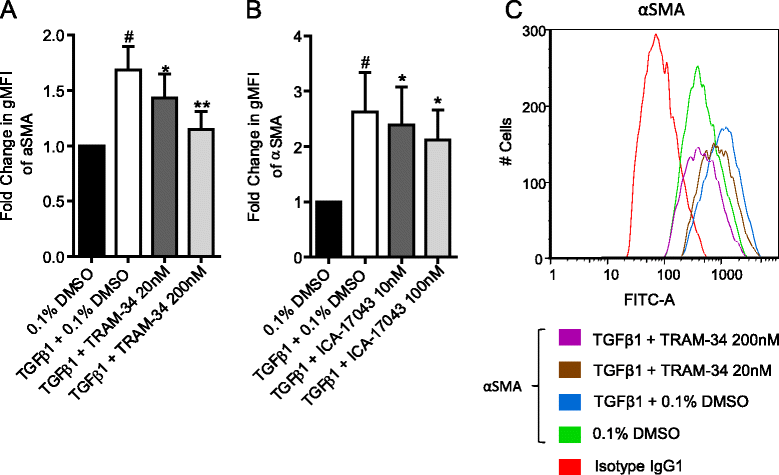
Background: Idiopathic pulmonary fibrosis (IPF) is a common and invariably lethal interstitial lung disease with poorly effective therapy. Blockade of the K(+) channel KCa3.1 reduces constitutive α-SMA and Smad2/3 nuclear translocation in IPF-derived human lung myofibroblasts (HLMFs), and inhibits several transforming growth factor beta 1 (TGFβ1)-dependent cell processes. We hypothesized that KCa3.1-dependent cell processes also regulate the TGFβ1-dependent Smad2/3 signalling pathway in HLMFs. HLMFs obtained from non-fibrotic controls (NFC) and IPF lungs were grown in vitro and examined for αSMA expression by immunofluorescence, RT-PCR, and flow cytometry. Two specific and distinct KCa3.1 blockers (TRAM-34 200 nM and ICA-17043 [Senicapoc] 100 nM) were used to determine their effects on TGFβ1-dependent signalling. Expression of phosphorylated and total Smad2/3 following TGFβ1 stimulation was determined by Western blot and Smad2/3 nuclear translocation by immunofluorescence.
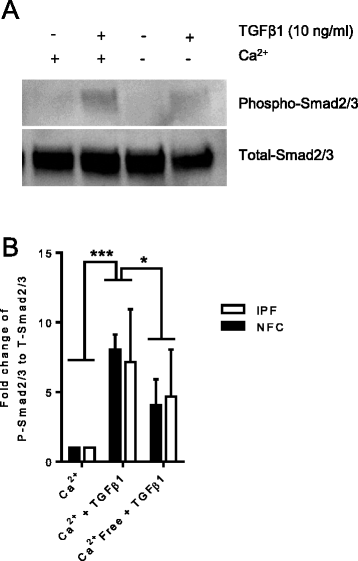
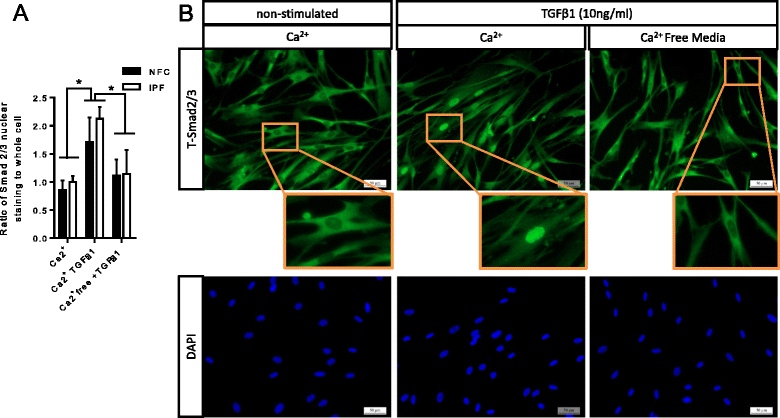
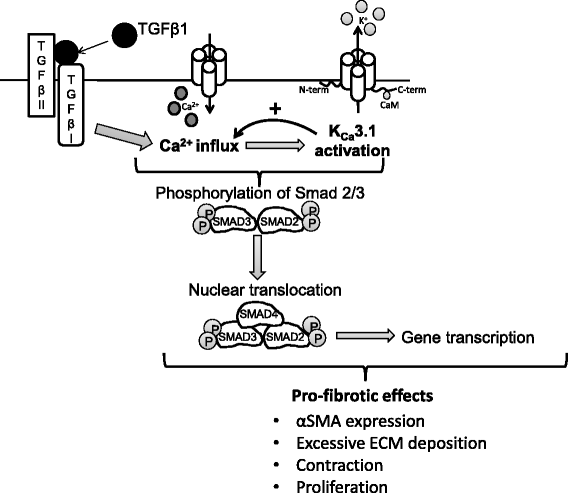
Results: KCa3.1 block attenuated TGFβ1-dependent Smad2/3 phosphorylation and nuclear translocation, and this was mimicked by lowering the extracellular Ca(2+) concentration. KCa3.1 block also inhibited Smad2/3-dependent gene transcription (αSMA, collagen type I), inhibited KCa3.1 mRNA expression, and attenuated TGFβ1-dependent αSMA protein expression.
Conclusions: KCa3.1 activity regulates TGFβ1-dependent effects in NFC- and IPF-derived primary HLMFs through the regulation of the TGFβ1/Smad signalling pathway, with promotion of downstream gene transcription and protein expression. KCa3.1 blockers may offer a novel approach to treating IPF.
Keywords: Human lung myofibroblast; Idiopathic pulmonary fibrosis; Potassium channel KCa3.1.
Figures
Similar articles
-
Blockade of KCa3.1: A novel target to treat TGF-β1 induced conjunctival fibrosis.Exp Eye Res. 2018 Feb;167:140-144. doi: 10.1016/j.exer.2017.12.003. Epub 2017 Dec 12. Exp Eye Res. 2018. PMID: 29242028 Free PMC article.
-
Increased constitutive αSMA and Smad2/3 expression in idiopathic pulmonary fibrosis myofibroblasts is KCa3.1-dependent.Respir Res. 2014 Dec 5;15(1):155. doi: 10.1186/s12931-014-0155-5. Respir Res. 2014. PMID: 25476248 Free PMC article.
-
The K+ channel KCa3.1 as a novel target for idiopathic pulmonary fibrosis.PLoS One. 2013 Dec 31;8(12):e85244. doi: 10.1371/journal.pone.0085244. eCollection 2013. PLoS One. 2013. PMID: 24392001 Free PMC article.
-
Lipoxin A4 Attenuates Constitutive and TGF-β1-Dependent Profibrotic Activity in Human Lung Myofibroblasts.J Immunol. 2015 Sep 15;195(6):2852-60. doi: 10.4049/jimmunol.1500936. Epub 2015 Aug 14. J Immunol. 2015. PMID: 26276873 Free PMC article.
-
Ca2+-Activated K+ Channel KCa3.1 as a Therapeutic Target for Immune Disorders.Biol Pharm Bull. 2018;41(8):1158-1163. doi: 10.1248/bpb.b18-00078. Biol Pharm Bull. 2018. PMID: 30068864 Review.
Cited by
-
Hyperosmolar potassium inhibits myofibroblast conversion and reduces scar tissue formation.ACS Biomater Sci Eng. 2019 Oct 14;5(10):5327-5336. doi: 10.1021/acsbiomaterials.9b00810. Epub 2019 Sep 18. ACS Biomater Sci Eng. 2019. PMID: 32440531 Free PMC article.
-
Blockade of KCa3.1: A novel target to treat TGF-β1 induced conjunctival fibrosis.Exp Eye Res. 2018 Feb;167:140-144. doi: 10.1016/j.exer.2017.12.003. Epub 2017 Dec 12. Exp Eye Res. 2018. PMID: 29242028 Free PMC article.
-
Ca2+ signalling in fibroblasts and the therapeutic potential of KCa3.1 channel blockers in fibrotic diseases.Br J Pharmacol. 2020 Mar;177(5):1003-1024. doi: 10.1111/bph.14939. Epub 2020 Feb 3. Br J Pharmacol. 2020. PMID: 31758702 Free PMC article. Review.
-
A model of human lung fibrogenesis for the assessment of anti-fibrotic strategies in idiopathic pulmonary fibrosis.Sci Rep. 2018 Jan 10;8(1):342. doi: 10.1038/s41598-017-18555-9. Sci Rep. 2018. PMID: 29321510 Free PMC article.
-
Evaluation of Pirfenidone and Nintedanib in a Human Lung Model of Fibrogenesis.Front Pharmacol. 2021 Oct 12;12:679388. doi: 10.3389/fphar.2021.679388. eCollection 2021. Front Pharmacol. 2021. PMID: 34712131 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

