Capsules, toxins and AtxA as virulence factors of emerging Bacillus cereus biovar anthracis
- PMID: 25830379
- PMCID: PMC4382292
- DOI: 10.1371/journal.pntd.0003455
Capsules, toxins and AtxA as virulence factors of emerging Bacillus cereus biovar anthracis
Erratum in
-
Correction: Capsules, toxins and AtxA as virulence factors of emerging Bacillus cereus biovar anthracis.PLoS Negl Trop Dis. 2015 Apr 22;9(4):e0003746. doi: 10.1371/journal.pntd.0003746. eCollection 2015 Apr. PLoS Negl Trop Dis. 2015. PMID: 25902046 Free PMC article.
Abstract
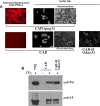
Emerging B. cereus strains that cause anthrax-like disease have been isolated in Cameroon (CA strain) and Côte d'Ivoire (CI strain). These strains are unusual, because their genomic characterisation shows that they belong to the B. cereus species, although they harbour two plasmids, pBCXO1 and pBCXO2, that are highly similar to the pXO1 and pXO2 plasmids of B. anthracis that encode the toxins and the polyglutamate capsule respectively. The virulence factors implicated in the pathogenicity of these B. cereus bv anthracis strains remain to be characterised. We tested their virulence by cutaneous and intranasal delivery in mice and guinea pigs; they were as virulent as wild-type B. anthracis. Unlike as described for pXO2-cured B. anthracis, the CA strain cured of the pBCXO2 plasmid was still highly virulent, showing the existence of other virulence factors. Indeed, these strains concomitantly expressed a hyaluronic acid (HA) capsule and the B. anthracis polyglutamate (PDGA) capsule. The HA capsule was encoded by the hasACB operon on pBCXO1, and its expression was regulated by the global transcription regulator AtxA, which controls anthrax toxins and PDGA capsule in B. anthracis. Thus, the HA and PDGA capsules and toxins were co-regulated by AtxA. We explored the respective effect of the virulence factors on colonisation and dissemination of CA within its host by constructing bioluminescent mutants. Expression of the HA capsule by itself led to local multiplication and, during intranasal infection, to local dissemination to the adjacent brain tissue. Co-expression of either toxins or PDGA capsule with HA capsule enabled systemic dissemination, thus providing a clear evolutionary advantage. Protection against infection by B. cereus bv anthracis required the same vaccination formulation as that used against B. anthracis. Thus, these strains, at the frontier between B. anthracis and B. cereus, provide insight into how the monomorphic B. anthracis may have emerged.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Jensen GB, Hansen BM, Eilenberg J, Mahillon J (2003) The hidden lifestyles of Bacillus cereus and relatives. Environ Microbiol 5: 631–640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

