A systematic review of the efficacy and safety experience reported for sorafenib in advanced renal cell carcinoma (RCC) in the post-approval setting
- PMID: 25830512
- PMCID: PMC4382117
- DOI: 10.1371/journal.pone.0120877
A systematic review of the efficacy and safety experience reported for sorafenib in advanced renal cell carcinoma (RCC) in the post-approval setting
Abstract
Background: Sorafenib was FDA approved in 2005 for treatment of renal cell carcinoma (RCC) based on the results of the pivotal phase 3 clinical trial, TARGET (Treatment Approaches in Renal Cancer Global Evaluation Trial). Since that time, numerous clinical studies have been undertaken that substantially broaden our knowledge of the use of sorafenib for this indication.
Methods: We systematically reviewed PubMed, Web of Science, Embase, Cochrane Library, and www.clinicaltrials.gov for prospective clinical studies using single agent sorafenib in RCC and published since 2005. Primary endpoints of interest were progression-free survival (PFS) and safety. PROSPERO International prospective register of systematic reviews #CRD42014010765.
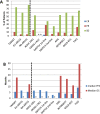
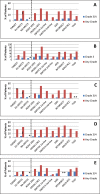
Results: We identified 30 studies in which 2182 patients were treated with sorafenib, including 1575 patients who participated in randomized controlled phase 3 trials. In these trials, sorafenib was administered as first-, second- or third-line treatment. Heterogeneity among trial designs and reporting of data precluded statistical comparisons among trials or with TARGET. The PFS appeared shorter in second- vs. first-line treatment, consistent with the more advanced tumor status in the second-line setting. In some trials, incidences of grade 3/4 hypertension or hand-foot skin reaction (HFSR) were more than double that seen in TARGET (4% and 6%, respectively). These variances may be attributable to increased recognition of HFSR, or potentially differences in dose adjustments, that could be consequences of increased familiarity with sorafenib usage. Several small studies enrolled exclusively Asian patients. These studies reported notably longer PFS than was observed in TARGET. However, no obvious corresponding differences in disease control rate and overall survival were seen.
Conclusions: Collectively, more recent experiences using sorafenib in RCC are consistent with results reported for TARGET with no marked changes of response endpoints or new safety signals observed.
Conflict of interest statement
Figures

References
-
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356: 125–134. - PubMed
-
- Beck J, Procopio G, Bajetta E, Keilholz U, Negrier S, Szczylik C, et al. Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) expanded-access study: a large open-label study in diverse community settings. Ann Oncol. 2011;22: 1812–1823. 10.1093/annonc/mdq651 - DOI - PubMed
-
- Beck J, Bajetta E, Escudier B, Negrier S, Keilholz U, Szczylik C, et al. A large open-label, non-comparative, phase III study of the multi-targeted kinase inhibitor sorafenib in European patients with advanced renal cell carcinoma. Eur J Cancer Suppl. 2007;5: 300: abstract 4506.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

