B7H6-specific chimeric antigen receptors lead to tumor elimination and host antitumor immunity
- PMID: 25830550
- PMCID: PMC4529373
- DOI: 10.1038/gt.2015.29
B7H6-specific chimeric antigen receptors lead to tumor elimination and host antitumor immunity
Abstract
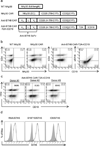
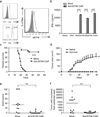
Chimeric antigen receptor (CAR) T-cell therapies have demonstrated durable and potentially curative therapeutic efficacy against B-cell leukemia in clinical trials. A CAR strategy can target any tumor surface antigens as long as an antigen-binding receptor can be generated. New CARs that target solid tumors and have the potential to target multiple tumor types are needed. In this study, B7H6, a ligand for the NK cell activating receptor NKp30, was targeted to create a CAR that targets multiple tumor types. B7H6 is expressed on various primary human tumors, including leukemia, lymphoma and gastrointestinal stromal tumors, but it is not constitutively expressed on normal tissues. B7H6-specific CAR T cells have robust cellular cytotoxicity and interferon-γ secretion when co-cultured with B7H6+ tumor cells, and they exhibit little self-reactivity to immature dendritic cells or pro-inflammatory monocytes. In vivo, B7H6-specific CAR T cells greatly enhanced the survival of RMA/B7H6 lymphoma-bearing mice. The long-term survivor mice were protected against a B7H6-deficient tumor re-challenge. This CAR therapy also decreased tumor burden in a murine ovarian cancer model. In conclusion, B7H6-specific CARs have the potential to treat B7H6+ hematologic and solid tumors.
Conflict of interest statement
Ming-Ru Wu and Leslie DeMars declare no potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

