Fatty acid carbon is essential for dNTP synthesis in endothelial cells
- PMID: 25830893
- PMCID: PMC4413024
- DOI: 10.1038/nature14362
Fatty acid carbon is essential for dNTP synthesis in endothelial cells
Erratum in
-
Corrigendum: Fatty acid carbon is essential for dNTP synthesis in endothelial cells.Nature. 2015 Oct 1;526(7571):144. doi: 10.1038/nature14624. Epub 2015 Aug 5. Nature. 2015. PMID: 26245368 No abstract available.
Abstract
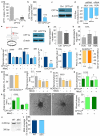
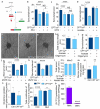
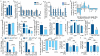
The metabolism of endothelial cells during vessel sprouting remains poorly studied. Here we report that endothelial loss of CPT1A, a rate-limiting enzyme of fatty acid oxidation (FAO), causes vascular sprouting defects due to impaired proliferation, not migration, of human and murine endothelial cells. Reduction of FAO in endothelial cells did not cause energy depletion or disturb redox homeostasis, but impaired de novo nucleotide synthesis for DNA replication. Isotope labelling studies in control endothelial cells showed that fatty acid carbons substantially replenished the Krebs cycle, and were incorporated into aspartate (a nucleotide precursor), uridine monophosphate (a precursor of pyrimidine nucleoside triphosphates) and DNA. CPT1A silencing reduced these processes and depleted endothelial cell stores of aspartate and deoxyribonucleoside triphosphates. Acetate (metabolized to acetyl-CoA, thereby substituting for the depleted FAO-derived acetyl-CoA) or a nucleoside mix rescued the phenotype of CPT1A-silenced endothelial cells. Finally, CPT1 blockade inhibited pathological ocular angiogenesis in mice, suggesting a novel strategy for blocking angiogenesis.
Figures



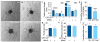



Comment in
-
Metabolism: Growth in the fat lane.Nature. 2015 Apr 9;520(7546):165-6. doi: 10.1038/nature14375. Epub 2015 Apr 1. Nature. 2015. PMID: 25830890 No abstract available.
References
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. - PubMed
-
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. - PubMed
-
- De Bock K, et al. Role of PFKFB3-Driven Glycolysis in Vessel Sprouting. Cell. 2013;154:651–663. - PubMed
-
- De Bock K, Georgiadou M, Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013;18:634–647. - PubMed
References unique to the Methods section
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

