A novel Werner Syndrome mutation: pharmacological treatment by read-through of nonsense mutations and epigenetic therapies
- PMID: 25830902
- PMCID: PMC4622951
- DOI: 10.1080/15592294.2015.1027853
A novel Werner Syndrome mutation: pharmacological treatment by read-through of nonsense mutations and epigenetic therapies
Abstract
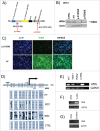
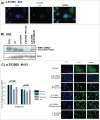
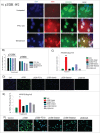
Werner Syndrome (WS) is a rare inherited disease characterized by premature aging and increased propensity for cancer. Mutations in the WRN gene can be of several types, including nonsense mutations, leading to a truncated protein form. WRN is a RecQ family member with both helicase and exonuclease activities, and it participates in several cell metabolic pathways, including DNA replication, DNA repair, and telomere maintenance. Here, we reported a novel homozygous WS mutation (c.3767 C > G) in 2 Argentinian brothers, which resulted in a stop codon and a truncated protein (p.S1256X). We also observed increased WRN promoter methylation in the cells of patients and decreased messenger WRN RNA (WRN mRNA) expression. Finally, we showed that the read-through of nonsense mutation pharmacologic treatment with both aminoglycosides (AGs) and ataluren (PTC-124) in these cells restores full-length protein expression and WRN functionality.
Keywords: Epigenetics; PTC read-through therapy; Werner Syndrome; methylation; mutation.
Figures
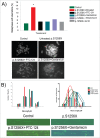
References
-
- Epstein CJ, Martin GM, Schultz AL, Motulsky AG. Werner's syndrome a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore) 1966; 45:177-21; PMID:5327241 - PubMed
-
- Salk D. Werner's syndrome: a review of recent research with an analysis of connective tissue metabolism, growth control of cultured cells, and chromosomal aberrations. Hum Genet 1982; 62:1-5; PMID:6759366; http://dx.doi.org/10.1007/BF00295598 - DOI - PubMed
-
- Goto M. Hierarchical deterioration of body systems in Werner's syndrome: implications for normal ageing. Mech Ageing Dev 1997; 98:239-54; PMID:9352493 - PubMed
-
- Lauper JM, Krause A, Vaughan TL, Monnat RJ, Jr. Spectrum and risk of neoplasia in Werner syndrome: a systematic review. PLoS One 2013; 8(4):e59709; PMID:23573208; http://dx.doi.org/10.1371/journal.pone.0059709 - DOI - PMC - PubMed
-
- Goto M, Miller RW, Ishikawa Y, Sugano H. Excess of rare cancers in Werner syndrome (adult progeria).Cancer Epidemiol Biomarkers Prev 1996; 5:239-46; PMID:8722214 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
