Evaluating outcomes used in cardiothoracic surgery interventional research: a systematic review of reviews to develop a core outcome set
- PMID: 25830921
- PMCID: PMC4382223
- DOI: 10.1371/journal.pone.0122204
Evaluating outcomes used in cardiothoracic surgery interventional research: a systematic review of reviews to develop a core outcome set
Abstract
Background: When planning clinical trials, it is a key element to choose appropriate outcomes that ensure the comparability of effects of interventions in ways that minimise bias. We hypothesise that outcome measures in cardiothoracic surgical trials are inconsistent and without standard. Therefore, comparing the relative effectiveness of interventions across studies is problematic. We surmise that cardiothoracic research has focused habitually on the identification of risk factors and on the reduction of adverse outcomes with less consideration of factors that contribute to well being and positive health outcomes (salutogenesis).
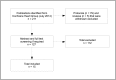
Methods and findings: We conducted a systematic review of reviews to determine both the type and number of outcomes reported in current cardiothoracic surgery interventional research, in order to identify a list of potential outcomes for a minimum core outcome set (COS). Special focus was placed on outcomes that emphasise salutogenesis. We interpreted salutogenic outcomes as those relating to optimum and/or positive health and well being. We searched Issue 7 (July 2014) of the Cochrane Database of Systematic Reviews. Systematic reviews of randomised trials on non-minimal-invasive off- or on-pump cardiothoracic surgery (elective and emergency, excluding transplants) investigating pre-, intra- or postsurgical interventions related to the outcome of the procedure were eligible for inclusion. We excluded protocols and withdrawn systematic reviews. Two review authors extracted outcome data independently. Unique lists of salutogenically and non-salutogenically focused outcomes were established. 15 systematic reviews involving 371 randomized trials and 58,253 patients were included in this review. Applied definitions of single and composite endpoints varied significantly, and patient-centred, salutogenically focused outcomes were seldom reported. One third of included reviews did not assess patient-centred outcomes at all; all other reviews were unable to perform meta-analyses due to an absence of data or heterogeneity in outcome measures. This compares to 36 non-salutogenically focused outcome domains representing 121 individual non-salutogenically focused outcomes, whereof 50% were assessed only once. Measures of mortality, cerebrovascular complications and hospitalisation were reported most frequently. Two reviews chose a composite endpoint as primary outcome. Pooled analysis of composite endpoints was not possible, as the required data was not reported per patient in all components.
Conclusion: In cardiothoracic surgical trials, choice and definition of non-salutogenically focused single and composite outcomes are inconsistent. There is an absence of patient centred, salutogenically focused outcome parameters in cardiac trials. We recommend the development of a core outcome set of salutogenically focused and non-salutogenically focused outcomes for cardiothoracic surgical research.
Conflict of interest statement
Figures
Similar articles
-
Salutogenically focused outcomes in systematic reviews of intrapartum interventions: a systematic review of systematic reviews.Midwifery. 2014 Apr;30(4):e151-6. doi: 10.1016/j.midw.2013.11.002. Epub 2013 Nov 11. Midwifery. 2014. PMID: 24290422
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
Cited by
-
Inconsistent selection of outcomes and measurement devices found in shoulder arthroplasty research: An analysis of studies on ClinicalTrials.gov.PLoS One. 2017 Nov 10;12(11):e0187865. doi: 10.1371/journal.pone.0187865. eCollection 2017. PLoS One. 2017. PMID: 29125866 Free PMC article.
-
A core outcome set for adult cardiac surgery trials: A consensus study.PLoS One. 2017 Nov 2;12(11):e0186772. doi: 10.1371/journal.pone.0186772. eCollection 2017. PLoS One. 2017. PMID: 29095881 Free PMC article.
-
[Sense of Coherence Scale according to Antonovsky as a possible predictor for return to work for cardiac surgery intensive care patients].Anaesthesist. 2018 Jul;67(7):512-518. doi: 10.1007/s00101-018-0448-z. Epub 2018 May 14. Anaesthesist. 2018. PMID: 29761259 German.
-
Standardised Outcome Reporting for the Nutrition Management of Complex Chronic Disease: A Rapid Review.Nutrients. 2021 Sep 26;13(10):3388. doi: 10.3390/nu13103388. Nutrients. 2021. PMID: 34684389 Free PMC article. Review.
-
Comparison of Clinical Trial and Systematic Review Outcomes for the 4 Most Prevalent Eye Diseases.JAMA Ophthalmol. 2017 Sep 1;135(9):933-940. doi: 10.1001/jamaophthalmol.2017.2583. JAMA Ophthalmol. 2017. PMID: 28772305 Free PMC article.
References
-
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ. 2009;340(365): 1–10. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical