Current endoscopic ultrasound-guided approach to incidental subepithelial lesions: optimal or optional?
- PMID: 25830949
- PMCID: PMC4367205
Current endoscopic ultrasound-guided approach to incidental subepithelial lesions: optimal or optional?
Abstract
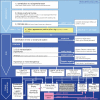
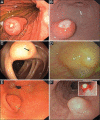
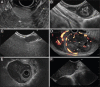
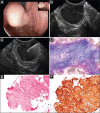
Subepithelial lesions (SEL) are identified during endoscopic procedures on a regular basis. They can occur anywhere in the gastrointestinal (GI) tract and are located beneath the normal epithelial layer, which explains why a tissue diagnosis is difficult to obtain with routine biopsies. Endoscopic ultrasound (EUS) is used to further characterize these lesions. EUS can distinguish intramural lesion from extramural compression. Furthermore, it allows allocation of intramural lesions to a specific layer of the GI wall and offers additional information as to whether a lesion could be benign or malignant. EUS also assists in choosing the optimal means of tissue acquisition. The choice of tissue acquisition is based on a number of factors, such as tumor size, EUS features, and location within the GI tract or within a specific layer of the GI wall. Furthermore, local expertise and patient factors should be considered when deciding whether tissue acquisition, surgical intervention or follow up is recommended. In this review we offer an EUS-guided approach to the evaluation of incidental SEL based on current evidence and point out areas of uncertainty, which explain why the proposed algorithmic approach may be optional rather than optimal.
Keywords: Endoscopic ultrasonography; fine needle aspiration; gastrointestinal stromal tumor; subepithelial lesions.
Conflict of interest statement
Conflict of Interest: None
Figures
References
-
- Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20–23. - PubMed
-
- Jenssen C, Dietrich CF. Endoscopic ultrasound of gastrointestinal subepithelial lesions. Ultraschall Med. 2008;29:236–256. - PubMed
-
- Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635–645. - PubMed
-
- Eckardt AJ, Wassef W. Diagnosis of subepithelial tumors in the GI tract. Endoscopy, EUS, and histology: bronze, silver, and gold standard? Gastrointest Endosc. 2005;62:209–212. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
