Aryl hydrocarbon receptor is necessary to protect fetal human pulmonary microvascular endothelial cells against hyperoxic injury: Mechanistic roles of antioxidant enzymes and RelB
- PMID: 25831079
- PMCID: PMC4458211
- DOI: 10.1016/j.taap.2015.03.023
Aryl hydrocarbon receptor is necessary to protect fetal human pulmonary microvascular endothelial cells against hyperoxic injury: Mechanistic roles of antioxidant enzymes and RelB
Abstract
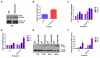
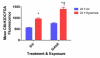
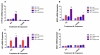
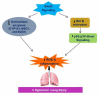
Hyperoxia contributes to the development of bronchopulmonary dysplasia (BPD) in premature infants. Activation of the aryl hydrocarbon receptor (AhR) protects adult and newborn mice against hyperoxic lung injury by mediating increases in the expression of phase I (cytochrome P450 (CYP) 1A) and phase II (NADP(H) quinone oxidoreductase (NQO1)) antioxidant enzymes (AOE). AhR positively regulates the expression of RelB, a component of the nuclear factor-kappaB (NF-κB) protein that contributes to anti-inflammatory processes in adult animals. Whether AhR regulates the expression of AOE and RelB, and protects fetal primary human lung cells against hyperoxic injury is unknown. Therefore, we tested the hypothesis that AhR-deficient fetal human pulmonary microvascular endothelial cells (HPMEC) will have decreased RelB activation and AOE, which will in turn predispose them to increased oxidative stress, inflammation, and cell death compared to AhR-sufficient HPMEC upon exposure to hyperoxia. AhR-deficient HPMEC showed increased hyperoxia-induced reactive oxygen species (ROS) generation, cleavage of poly(ADP-ribose) polymerase (PARP), and cell death compared to AhR-sufficient HPMEC. Additionally, AhR-deficient cell culture supernatants displayed increased macrophage inflammatory protein 1α and 1β, indicating a heightened inflammatory state. Interestingly, loss of AhR was associated with a significantly attenuated CYP1A1, NQO1, superoxide dismutase 1(SOD1), and nuclear RelB protein expression. These findings support the hypothesis that decreased RelB activation and AOE in AhR-deficient cells is associated with increased hyperoxic injury compared to AhR-sufficient cells.
Keywords: Antioxidant enzymes; Aryl hydrocarbon Receptor; Fetal human pulmonary microvascular endothelial cells; Hyperoxic injury; Oxidant stress; RelB.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





References
-
- Asikainen TM, White CW. Antioxidant defenses in the preterm lung: role for hypoxia-inducible factors in BPD? Toxicol Appl Pharmacol. 2005;203:177–188. - PubMed
-
- Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, Sime PJ. The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. The Journal of biological chemistry. 2008;283:28944–28957. - PMC - PubMed
-
- Barazzone C, White CW. Mechanisms of cell injury and death in hyperoxia: role of cytokines and Bcl-2 family proteins. Am J Respir Cell Mol Biol. 2000;22:517–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA125123/CA/NCI NIH HHS/United States
- HD-073323/HD/NICHD NIH HHS/United States
- R01 ES009132/ES/NIEHS NIH HHS/United States
- K08 HD073323/HD/NICHD NIH HHS/United States
- AI036211/AI/NIAID NIH HHS/United States
- R01 ES019689/ES/NIEHS NIH HHS/United States
- HL-087174/HL/NHLBI NIH HHS/United States
- ES-019689/ES/NIEHS NIH HHS/United States
- HL112516/HL/NHLBI NIH HHS/United States
- R01 HL087174/HL/NHLBI NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- R01 HL112516/HL/NHLBI NIH HHS/United States
- P30 AI036211/AI/NIAID NIH HHS/United States
- P30 ES023512/ES/NIEHS NIH HHS/United States
- ES-009132/ES/NIEHS NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- RR024574/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

