Modulation of orthogonal body waves enables high maneuverability in sidewinding locomotion
- PMID: 25831489
- PMCID: PMC4434722
- DOI: 10.1073/pnas.1418965112
Modulation of orthogonal body waves enables high maneuverability in sidewinding locomotion
Abstract
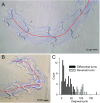
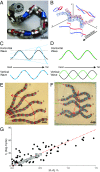
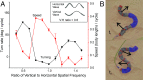
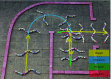
Many organisms move using traveling waves of body undulation, and most work has focused on single-plane undulations in fluids. Less attention has been paid to multiplane undulations, which are particularly important in terrestrial environments where vertical undulations can regulate substrate contact. A seemingly complex mode of snake locomotion, sidewinding, can be described by the superposition of two waves: horizontal and vertical body waves with a phase difference of ± 90°. We demonstrate that the high maneuverability displayed by sidewinder rattlesnakes (Crotalus cerastes) emerges from the animal's ability to independently modulate these waves. Sidewinder rattlesnakes used two distinct turning methods, which we term differential turning (26° change in orientation per wave cycle) and reversal turning (89°). Observations of the snakes suggested that during differential turning the animals imposed an amplitude modulation in the horizontal wave whereas in reversal turning they shifted the phase of the vertical wave by 180°. We tested these mechanisms using a multimodule snake robot as a physical model, successfully generating differential and reversal turning with performance comparable to that of the organisms. Further manipulations of the two-wave system revealed a third turning mode, frequency turning, not observed in biological snakes, which produced large (127°) in-place turns. The two-wave system thus functions as a template (a targeted motor pattern) that enables complex behaviors in a high-degree-of-freedom system to emerge from relatively simple modulations to a basic pattern. Our study reveals the utility of templates in understanding the control of biological movement as well as in developing control schemes for limbless robots.
Keywords: biomechanics; control; robotics; sidewinder; template.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Snake robot uncovers secrets to sidewinders' maneuverability.Proc Natl Acad Sci U S A. 2015 May 12;112(19):5870-1. doi: 10.1073/pnas.1504307112. Epub 2015 May 1. Proc Natl Acad Sci U S A. 2015. PMID: 25934919 Free PMC article. No abstract available.
References
-
- Webb PW, Blake RW. 1985. Swimming. Functional Vertebrate Morphology, eds Hildebrand M, Bramble DM, Liem KF, Wake DB (Harvard Univ Press, Cambridge, MA), pp 110–128.
-
- Taylor G. Analysis of the swimming of long and narrow animals. Proc R Soc Lond A Math Phys Sci. 1952;214(1117):158–183.
-
- Jayne BC. Kinematics of terrestrial snake locomotion. Copeia. 1986;1986:195–208.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

