Immunogenicity of reduced dose priming schedules of serogroup C meningococcal conjugate vaccine followed by booster at 12 months in infants: open label randomised controlled trial
- PMID: 25832102
- PMCID: PMC4382115
- DOI: 10.1136/bmj.h1554
Immunogenicity of reduced dose priming schedules of serogroup C meningococcal conjugate vaccine followed by booster at 12 months in infants: open label randomised controlled trial
Erratum in
-
Immunogenicity of reduced dose priming schedules of serogroup C meningococcal conjugate vaccine followed by booster at 12 months in infants: open label randomised controlled trial.BMJ. 2016 May 6;353:i2605. doi: 10.1136/bmj.i2605. BMJ. 2016. PMID: 27153852 No abstract available.
Abstract
Objective: To determine whether the immunogenicity of a single dose infant priming schedule of serogroup C meningococcal (MenC) conjugate vaccine is non-inferior to a two dose priming schedule when followed by a booster dose at age 12 months.
Design: Phase IV open label randomised controlled trial carried out from July 2010 until August 2013 SETTING: Four centres in the United Kingdom and one centre in Malta.
Participants: Healthy infants aged 6-12 weeks followed up until age 24 months.
Interventions: In the priming phase of the trial 509 infants were randomised in a 10:10:7:4 ratio into four groups to receive either a single MenC-cross reacting material 197 (CRM) dose at 3 months; two doses of MenC-CRM at 3 and 4 months; a single MenC-polysaccharide-tetanus toxoid (TT) dose at 3 months; or no MenC doses, respectively. Haemophilus influenzae type b (Hib)-MenC-TT vaccine was administered to all infants at 12 months of age. All infants also received the nationally routinely recommended vaccines. Blood samples were taken at age 5, 12, 13, and 24 months.
Main outcome measure: MenC serum bactericidal antibody assay with rabbit complement (rSBA) one month after the Hib-MenC-TT vaccine. Non-inferiority was met if the lower 95% confidence limit of the difference in the mean log10 MenC rSBA between the single dose MenC-CRM and the two dose MenC-CRM groups was >-0.35.
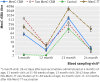
Results: The primary objective was met: after a Hib-MenC-TT booster dose at 12 months of age the MenC rSBA geometric mean titres induced in infants primed with a single MenC-CRM dose were not inferior to those induced in participants primed with two MenC-CRM doses in infancy (660 (95% confidence interval 498 to 876) v 295 (220 to 398)) with a corresponding difference in the mean log10 MenC rSBA of 0.35 (0.17 to 0.53) that showed superiority of the single over the two dose schedule). Exploration of differences between the priming schedules showed that one month after Hib-MenC-TT vaccination, MenC rSBA ≥ 1:8 was observed in >96% of participants previously primed with any of the MenC vaccine schedules in infancy and in 83% of those who were not vaccinated against MenC in infancy. The MenC rSBA geometric mean titres induced by the Hib-MenC-TT boost were significantly higher in children who were primed with one rather than two MenC-CRM doses in infancy. Only priming with MenC-TT, however, induced robust MenC bactericidal antibody after the Hib-MenC-TT booster that persisted until 24 months of age.
Conclusions: MenC vaccination programmes with two MenC infant priming doses could be reduced to a single priming dose without reducing post-boost antibody titres. When followed by a Hib-MenC-TT booster dose, infant priming with a single MenC-TT vaccine dose induces a more robust antibody response than one or two infant doses of MenC-CRM. Bactericidal antibody induced by a single Hib-MenC-TT conjugate vaccine dose at 12 months of age (that is, a toddler only schedule), without infant priming, is not well sustained at 24 months. Because of rapid waning of MenC antibody, programmes using toddler only schedules will still need to rely on herd protection to protect infants and young children.Trial registration Eudract No: 2009-016579-31; NCT01129518; study ID: 2008_06 (http://clinicaltrials.gov).
© Pace et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Similar articles
-
A novel combined Hib-MenC-TT glycoconjugate vaccine as a booster dose for toddlers: a phase 3 open randomised controlled trial.Arch Dis Child. 2008 Nov;93(11):963-70. doi: 10.1136/adc.2007.136036. Epub 2008 May 7. Arch Dis Child. 2008. PMID: 18463125 Clinical Trial.
-
Use of a booster dose of capsular group C meningococcal glycoconjugate vaccine to demonstrate immunologic memory in children primed with one or two vaccine doses in infancy.Vaccine. 2016 Dec 7;34(50):6350-6357. doi: 10.1016/j.vaccine.2016.10.038. Epub 2016 Oct 28. Vaccine. 2016. PMID: 28029540 Clinical Trial.
-
Five-year antibody persistence and safety following a booster dose of combined Haemophilus influenzae type b-Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine.Pediatr Infect Dis J. 2012 Oct;31(10):1074-7. doi: 10.1097/INF.0b013e318269433a. Pediatr Infect Dis J. 2012. PMID: 22828645 Clinical Trial.
-
Is a single infant priming dose of meningococcal serogroup C conjugate vaccine in the United Kingdom sufficient?Hum Vaccin Immunother. 2015;11(6):1501-6. doi: 10.1080/21645515.2015.1019189. Hum Vaccin Immunother. 2015. PMID: 25912095 Free PMC article. Review.
-
Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV).Pediatr Infect Dis J. 2009 Apr;28(4 Suppl):S97-S108. doi: 10.1097/INF.0b013e318199f61b. Pediatr Infect Dis J. 2009. PMID: 19325452 Review.
Cited by
-
Vaccine Adjuvants Differentially Affect Kinetics of Antibody and Germinal Center Responses.Front Immunol. 2020 Sep 23;11:579761. doi: 10.3389/fimmu.2020.579761. eCollection 2020. Front Immunol. 2020. PMID: 33072125 Free PMC article.
-
A systems approach to vaccine decision making.Vaccine. 2017 Jan 20;35 Suppl 1(Suppl 1):A36-A42. doi: 10.1016/j.vaccine.2016.11.033. Epub 2016 Dec 22. Vaccine. 2017. PMID: 28017430 Free PMC article.
-
Mice Immunized with the Vaccine Candidate HexaPro Spike Produce Neutralizing Antibodies against SARS-CoV-2.Vaccines (Basel). 2021 May 12;9(5):498. doi: 10.3390/vaccines9050498. Vaccines (Basel). 2021. PMID: 34066016 Free PMC article.
-
Research quality and dissemination of paediatric randomised controlled trials with and without patient and family engagement: systematic review.BMJ Open. 2025 Mar 12;15(3):e086934. doi: 10.1136/bmjopen-2024-086934. BMJ Open. 2025. PMID: 40074267 Free PMC article.
-
The epidemiology of invasive meningococcal disease and the utility of vaccination in Malta.Eur J Clin Microbiol Infect Dis. 2020 Oct;39(10):1885-1897. doi: 10.1007/s10096-020-03914-8. Epub 2020 May 16. Eur J Clin Microbiol Infect Dis. 2020. PMID: 32418063 Free PMC article.
References
-
- Borrow R, Abad R, Trotter C, et al. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine 2013;31:4477-86. - PubMed
-
- Campbell H, Borrow R, Salisbury D, et al. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine 2009. Jun 24;27(suppl 2):B20-9. - PubMed
-
- Richmond P, Borrow R, Miller E, et al. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J Infect Dis 1999;179:1569-72. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
