Crystal structures of Mycobacterial MeaB and MMAA-like GTPases
- PMID: 25832174
- PMCID: PMC4631608
- DOI: 10.1007/s10969-015-9197-2
Crystal structures of Mycobacterial MeaB and MMAA-like GTPases
Abstract
The methylmalonyl Co-A mutase-associated GTPase MeaB from Methylobacterium extorquens is involved in glyoxylate regulation and required for growth. In humans, mutations in the homolog methylmalonic aciduria associated protein (MMAA) cause methylmalonic aciduria, which is often fatal. The central role of MeaB from bacteria to humans suggests that MeaB is also important in other, pathogenic bacteria such as Mycobacterium tuberculosis. However, the identity of the mycobacterial MeaB homolog is presently unclear. Here, we identify the M. tuberculosis protein Rv1496 and its homologs in M. smegmatis and M. thermoresistibile as MeaB. The crystal structures of all three homologs are highly similar to MeaB and MMAA structures and reveal a characteristic three-domain homodimer with GDP bound in the G domain active site. A structure of Rv1496 obtained from a crystal grown in the presence of GTP exhibited electron density for GDP, suggesting GTPase activity. These structures identify the mycobacterial MeaB and provide a structural framework for therapeutic targeting of M. tuberculosis MeaB.
Figures



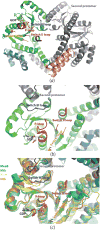
References
-
- Korotkova N, Lidstrom ME. MeaB is a component of the methylmalonyl-CoA mutase complex required for protection of the enzyme from inactivation. J Biol Chem. 2004;279:13652–13658. - PubMed
-
- Dobson CM, Wai T, Leclerc D, Kadir H, Narang M, Lerner-Ellis JP, Hudson TJ, Rosenblatt DS, Gravel RA. Identification of the gene responsible for the cblB complementation group of vitamin B12-dependent methylmalonic aciduria. Hum Mol Genet. 2002;11:3361–3369. - PubMed
-
- Hubbard PA, Padovani D, Labunska T, Mahlstedt SA, Banerjee R, Drennan CL. Crystal structure and mutagenesis of the metallochaperone MeaB: insight into the causes of methylmalonic aciduria. J Biol Chem. 2007;282:31308–31316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

