Successful Combination of Nucleic Acid Amplification Test Diagnostics and Targeted Deferred Neisseria gonorrhoeae Culture
- PMID: 25832300
- PMCID: PMC4432034
- DOI: 10.1128/JCM.00369-15
Successful Combination of Nucleic Acid Amplification Test Diagnostics and Targeted Deferred Neisseria gonorrhoeae Culture
Abstract
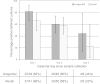
Nucleic acid amplification tests (NAATs) are recommended for the diagnosis of N. gonorrhoeae infections because of their superior sensitivity. Increasing NAAT use causes a decline in crucial antimicrobial resistance (AMR) surveillance data, which rely on culture. We analyzed the suitability of the ESwab system for NAAT diagnostics and deferred targeted N. gonorrhoeae culture to allow selective and efficient culture based on NAAT results. We included patients visiting the STI Clinic Amsterdam, The Netherlands, in 2013. Patient characteristics and urogenital and rectal samples for direct N. gonorrhoeae culture, standard NAAT, and ESwab were collected. Standard NAAT and NAAT on ESwab samples were performed using the Aptima Combo 2 assay for N. gonorrhoeae and C. trachomatis. Two deferred N. gonorrhoeae cultures were performed on NAAT-positive ESwab samples after storage at 4°C for 1 to 3 days. We included 2,452 samples from 1,893 patients. In the standard NAAT, 107 samples were N. gonorrhoeae positive and 284 were C. trachomatis positive. The sensitivities of NAAT on ESwab samples were 83% (95% confidence interval [CI], 75 to 90%) and 87% (95% CI, 82 to 90%), respectively. ESwab samples were available for 98 of the gonorrhea-positive samples. Of these, 82% were positive in direct culture and 69% and 56% were positive in the 1st and 2nd deferred cultures, respectively (median storage times, 27 and 48 h, respectively). Deferred culture was more often successful in urogenital samples or when the patient had symptoms at the sampling site. Deferred N. gonorrhoeae culture of stored ESwab samples is feasible and enables AMR surveillance. To limit the loss in NAAT sensitivity, we recommend obtaining separate samples for NAAT and deferred culture.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- World Health Organization. 2011. Emergence of multi-drug resistant Neisseria gonorrhoeae–threat of global rise in untreatable sexually transmitted infections. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/hq/2011/WHO_RHR_11.14_eng.pdf.
-
- World Health Organization. 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2012/9789241503501_eng.pdf.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

