PSF: nuclear busy-body or nuclear facilitator?
- PMID: 25832716
- PMCID: PMC4478221
- DOI: 10.1002/wrna.1280
PSF: nuclear busy-body or nuclear facilitator?
Abstract
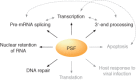
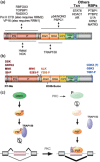
PTB-associated splicing factor (PSF) is an abundant and essential nucleic acid-binding protein that participates in a wide range of gene regulatory processes and cellular response pathways. At the protein level, PSF consists of multiple domains, many of which remain poorly characterized. Although grouped in a family with the proteins p54nrb/NONO and PSPC1 based on sequence homology, PSF contains additional protein sequence not included in other family members. Consistently, PSF has also been implicated in functions not ascribed to p54nrb/NONO or PSPC1. Here, we provide a review of the cellular activities in which PSF has been implicated and what is known regarding the mechanisms by which PSF functions in each case. We propose that the complex domain arrangement of PSF allows for its diversity of function and integration of activities. Finally, we discuss recent evidence that individual activities of PSF can be regulated independently from one another through the activity of domain-specific co-factors.
© 2015 The Authors. WIREs RNA published by John Wiley & Sons, Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

