Preference for concentric orientations in the mouse superior colliculus
- PMID: 25832803
- PMCID: PMC4396361
- DOI: 10.1038/ncomms7773
Preference for concentric orientations in the mouse superior colliculus
Abstract
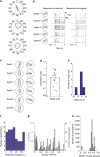
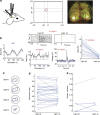
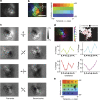
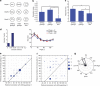
The superior colliculus is a layered structure important for body- and gaze-orienting responses. Its superficial layer is, next to the lateral geniculate nucleus, the second major target of retinal ganglion axons and is retinotopically organized. Here we show that in the mouse there is also a precise organization of orientation preference. In columns perpendicular to the tectal surface, neurons respond to the same visual location and prefer gratings of the same orientation. Calcium imaging and extracellular recording revealed that the preferred grating varies with retinotopic location, and is oriented parallel to the concentric circle around the centre of vision through the receptive field. This implies that not all orientations are equally represented across the visual field. This makes the superior colliculus different from visual cortex and unsuitable for translation-invariant object recognition and suggests that visual stimuli might have different behavioural consequences depending on their retinotopic location.
Figures

References
-
- Hubel D. H. & Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc. R. Soc. Lond. B Biol. Sci. 198, 1–59 (1977) . - PubMed
-
- Ohki K., Chung S., Ch'ng Y. H., Kara P. & Reid R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005) . - PubMed
-
- Hofbauer A. & Dräger U. C. Depth segregation of retinal ganglion cells projecting to mouse superior colliculus. J. Comp. Neurol. 234, 465–474 (1985) . - PubMed
-
- Dräger U. C. & Hubel D. H. Physiology of visual cells in mouse superior colliculus and correlation with somatosensory and auditory input. Nature 253, 203–204 (1975) . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

