Synergism of ursolic acid derivative US597 with 2-deoxy-D-glucose to preferentially induce tumor cell death by dual-targeting of apoptosis and glycolysis
- PMID: 25833312
- PMCID: PMC4650901
- DOI: 10.1038/srep05006
Synergism of ursolic acid derivative US597 with 2-deoxy-D-glucose to preferentially induce tumor cell death by dual-targeting of apoptosis and glycolysis
Abstract
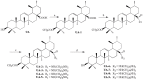
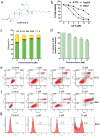
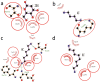
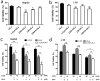
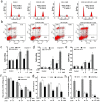
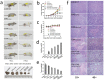
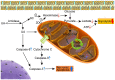
Ursolic acid (UA) is a naturally bioactive product that exhibits potential anticancer effects. The relatively safe and effective molecule intrigued us to explore a way to further improve its anti-cancer activity and tumor-targeting specificity. In the present study, a series of structural modifications of UA was achieved, which resulted in significant increase in growth inhibition on various cancer cell lines with minimal effects on normal cells. The leading molecule US597 (UA-4) caused depolarization of mitochondrial membrane potential, cell arrest in G0/G1 phase and apoptosis/necrosis in a dose-dependent manner. Structural docking suggested that the carbon chains of the modified UA derivatives compete strongly with glucose for binding to glucokinase, the key glycolysis enzyme presumably active in cancer cells. The combination of 2-deoxy-D-glucose (2-DG) and UA-4 induced cell cycle arrest in G2/M phase, promoted caspase-dependent cell death, reduced hexokinase activity, aggravated depletion of intracellular ATP, decreased lactate production and synergistically inhibited cancer cell growth in vitro (HepG2) and in vivo (H22). Collectively, our findings suggest that the structural modification enhances efficacy and selectivity of UA, and the combination of UA-4 with 2-DG produces synergistic inhibition on hepatoma cell proliferation by dual targeting of apoptosis and glycolysis.
Figures
Similar articles
-
UP12, a novel ursolic acid derivative with potential for targeting multiple signaling pathways in hepatocellular carcinoma.Biochem Pharmacol. 2015 Jan 15;93(2):151-62. doi: 10.1016/j.bcp.2014.11.014. Epub 2014 Dec 15. Biochem Pharmacol. 2015. PMID: 25522955
-
2-Deoxy-d-glucose Promotes Buforin IIb-Induced Cytotoxicity in Prostate Cancer DU145 Cells and Xenograft Tumors.Molecules. 2020 Dec 7;25(23):5778. doi: 10.3390/molecules25235778. Molecules. 2020. PMID: 33297583 Free PMC article.
-
Synthesis and Biological Evaluation of Novel Ursolic acid Derivatives as Potential Anticancer Prodrugs.Chem Biol Drug Des. 2015 Dec;86(6):1397-404. doi: 10.1111/cbdd.12608. Epub 2015 Jul 14. Chem Biol Drug Des. 2015. PMID: 26077799
-
Advances in the chemo‑preventive effects and mechanisms of ursolic acid against lung cancer (Review).Oncol Rep. 2025 Oct;54(4):126. doi: 10.3892/or.2025.8959. Epub 2025 Aug 1. Oncol Rep. 2025. PMID: 40747695 Free PMC article. Review.
-
Ursolic acid: a pentacyclic triterpenoid that exhibits anticancer therapeutic potential by modulating multiple oncogenic targets.Biotechnol Genet Eng Rev. 2023 Oct;39(2):729-759. doi: 10.1080/02648725.2022.2162257. Epub 2023 Jan 4. Biotechnol Genet Eng Rev. 2023. PMID: 36600517 Review.
Cited by
-
How Should the Worldwide Knowledge of Traditional Cancer Healing Be Integrated with Herbs and Mushrooms into Modern Molecular Pharmacology?Pharmaceuticals (Basel). 2022 Jul 14;15(7):868. doi: 10.3390/ph15070868. Pharmaceuticals (Basel). 2022. PMID: 35890166 Free PMC article. Review.
-
Anti-sickling Activity of Ursolic Acid Isolated from the Leaves of Ocimum gratissimum L. (Lamiaceae).Nat Prod Bioprospect. 2015 Aug;5(4):215-21. doi: 10.1007/s13659-015-0070-6. Epub 2015 Sep 9. Nat Prod Bioprospect. 2015. PMID: 26351101 Free PMC article.
-
Mitochondria-targeting fluorescent molecules for high efficiency cancer growth inhibition and imaging.Chem Sci. 2019 Jul 8;10(34):7946-7951. doi: 10.1039/c9sc01410a. eCollection 2019 Sep 14. Chem Sci. 2019. PMID: 31853349 Free PMC article.
-
Non-thermal plasma with 2-deoxy-D-glucose synergistically induces cell death by targeting glycolysis in blood cancer cells.Sci Rep. 2015 Mar 4;5:8726. doi: 10.1038/srep08726. Sci Rep. 2015. PMID: 25735798 Free PMC article.
-
A pentacyclic triterpene natural product, ursolic acid and its prodrug US597 inhibit targets within cell adhesion pathway and prevent cancer metastasis.Oncotarget. 2015 Apr 20;6(11):9295-312. doi: 10.18632/oncotarget.3261. Oncotarget. 2015. PMID: 25823660 Free PMC article.
References
-
- Chattopadhyay D. et al. Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J Ethnopharmacol. 82, 229–237 (2002). - PubMed
-
- Lu J. et al. Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in mice. Brain Behav Immun. 25, 1658–1667 (2011). - PubMed
-
- Lee J. et al. Ursolic acid ameliorates thymic atrophy and hyperglycemia in streptozotocin–nicotinamide-induced diabetic mice. Chem-Biol Interact. 188, 635–642 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

