Pharmacist-led management of chronic pain in primary care: costs and benefits in a pilot randomised controlled trial
- PMID: 25833666
- PMCID: PMC4390732
- DOI: 10.1136/bmjopen-2014-006874
Pharmacist-led management of chronic pain in primary care: costs and benefits in a pilot randomised controlled trial
Abstract
Objectives: To explore differences in mean costs (from a UK National Health Service perspective) and effects of pharmacist-led management of chronic pain in primary care evaluated in a pilot randomised controlled trial (RCT), and to estimate optimal sample size for a definitive RCT.
Design: Regression analysis of costs and effects, using intention-to-treat and expected value of sample information analysis (EVSI).
Setting: Six general practices: Grampian (3); East Anglia (3).
Participants: 125 patients with complete resource use and short form-six-dimension questionnaire (SF-6D) data at baseline, 3 months and 6 months.
Interventions: Patients were randomised to either pharmacist medication review with face-to-face pharmacist prescribing or pharmacist medication review with feedback to general practitioner or treatment as usual (TAU).
Main outcome measures: Differences in mean total costs and effects measured as quality-adjusted life years (QALYs) at 6 months and EVSI for sample size calculation.
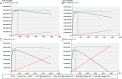
Results: Unadjusted total mean costs per patient were £452 for prescribing (SD: £466), £570 for review (SD: £527) and £668 for TAU (SD: £1333). After controlling for baseline costs, the adjusted mean cost differences per patient relative to TAU were £77 for prescribing (95% CI -82 to 237) and £54 for review (95% CI -103 to 212). Unadjusted mean QALYs were 0.3213 for prescribing (SD: 0.0659), 0.3161 for review (SD: 0.0684) and 0.3079 for TAU (SD: 0.0606). Relative to TAU, the adjusted mean differences were 0.0069 for prescribing (95% CI -0.0091 to 0.0229) and 0.0097 for review (95% CI -0.0054 to 0.0248). The EVSI suggested the optimal future trial size was between 460 and 690, and between 540 and 780 patients per arm using a threshold of £30,000 and £20,000 per QALY gained, respectively.
Conclusions: Compared with TAU, pharmacist-led interventions for chronic pain appear more costly and provide similar QALYs. However, these estimates are imprecise due to the small size of the pilot trial. The EVSI indicates that a larger trial is necessary to obtain more precise estimates of differences in mean effects and costs between treatment groups.
Trial registration number: ISRCTN06131530.
Keywords: HEALTH ECONOMICS; PAIN MANAGEMENT; PRIMARY CARE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- Scottish Intercollegiate Guidelines Network (SIGN). Management of chronic pain. Edinburgh: SIGN, 2013. (SIGN publication no. 136). http://www.sign.ac.uk
-
- Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986;3:S1–226. - PubMed
-
- Breivik H, Eisenberg E, O'Brien T, on behalf of OPENMinds. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care . BMC Public Health 2013;13:1229 10.1186/1471-2458-13-1229 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
