Centriolar satellite- and hMsd1/SSX2IP-dependent microtubule anchoring is critical for centriole assembly
- PMID: 25833712
- PMCID: PMC4472012
- DOI: 10.1091/mbc.E14-11-1561
Centriolar satellite- and hMsd1/SSX2IP-dependent microtubule anchoring is critical for centriole assembly
Abstract
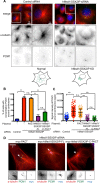
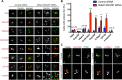
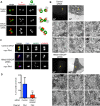
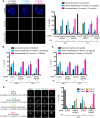
Centriolar satellites are numerous electron-dense granules dispersed around the centrosome. Mutations in their components are linked to various human diseases, but their molecular roles remain elusive. In particular, the significance of spatial communication between centriolar satellites and the centrosome is unknown. hMsd1/SSX2IP localizes to both the centrosome and centriolar satellites and is required for tethering microtubules to the centrosome. Here we show that hMsd1/SSX2IP-mediated microtubule anchoring is essential for proper centriole assembly and duplication. On hMsd1/SSX2IP knockdown, the centriolar satellites become stuck at the microtubule minus end near the centrosome. Intriguingly, these satellites contain many proteins that normally localize to the centrosome. Of importance, microtubule structures, albeit not being anchored properly, are still required for the emergence of abnormal satellites, as complete microtubule depolymerization results in the disappearance of these aggregates from the vicinity of the centrosome. We highlighted, using superresolution and electron microscopy, that under these conditions, centriole structures are faulty. Remarkably, these cells are insensitive to Plk4 overproduction-induced ectopic centriole formation, yet they accelerate centrosome reduplication upon hydroxyurea arrest. Finally, the appearance of satellite aggregates is cancer cell specific. Together our findings provide novel insights into the mechanism of centriole assembly and microtubule anchoring.
© 2015 Hori et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


Similar articles
-
The conserved Wdr8-hMsd1/SSX2IP complex localises to the centrosome and ensures proper spindle length and orientation.Biochem Biophys Res Commun. 2015 Dec 4-11;468(1-2):39-45. doi: 10.1016/j.bbrc.2015.10.169. Epub 2015 Nov 10. Biochem Biophys Res Commun. 2015. PMID: 26545777 Free PMC article.
-
Msd1/SSX2IP-dependent microtubule anchorage ensures spindle orientation and primary cilia formation.EMBO Rep. 2014 Feb;15(2):175-84. doi: 10.1002/embr.201337929. Epub 2014 Jan 7. EMBO Rep. 2014. PMID: 24397932 Free PMC article.
-
The centriolar satellite protein CCDC66 interacts with CEP290 and functions in cilium formation and trafficking.J Cell Sci. 2017 Apr 15;130(8):1450-1462. doi: 10.1242/jcs.196832. Epub 2017 Feb 24. J Cell Sci. 2017. PMID: 28235840 Free PMC article.
-
Regulation of centriolar satellite integrity and its physiology.Cell Mol Life Sci. 2017 Jan;74(2):213-229. doi: 10.1007/s00018-016-2315-x. Epub 2016 Aug 2. Cell Mol Life Sci. 2017. PMID: 27484406 Free PMC article. Review.
-
Accessorizing the centrosome: new insights into centriolar appendages and satellites.Curr Opin Struct Biol. 2021 Feb;66:148-155. doi: 10.1016/j.sbi.2020.10.021. Epub 2020 Dec 3. Curr Opin Struct Biol. 2021. PMID: 33279729 Review.
Cited by
-
A non-canonical function of Plk4 in centriolar satellite integrity and ciliogenesis through PCM1 phosphorylation.EMBO Rep. 2016 Mar;17(3):326-37. doi: 10.15252/embr.201541432. Epub 2016 Jan 11. EMBO Rep. 2016. PMID: 26755742 Free PMC article.
-
The conserved Wdr8-hMsd1/SSX2IP complex localises to the centrosome and ensures proper spindle length and orientation.Biochem Biophys Res Commun. 2015 Dec 4-11;468(1-2):39-45. doi: 10.1016/j.bbrc.2015.10.169. Epub 2015 Nov 10. Biochem Biophys Res Commun. 2015. PMID: 26545777 Free PMC article.
-
Selective autophagy maintains centrosome integrity and accurate mitosis by turnover of centriolar satellites.Nat Commun. 2019 Sep 13;10(1):4176. doi: 10.1038/s41467-019-12094-9. Nat Commun. 2019. PMID: 31519908 Free PMC article.
-
The impact of chromosomal translocation locus and fusion oncogene coding sequence in synovial sarcomagenesis.Oncogene. 2016 Sep 22;35(38):5021-32. doi: 10.1038/onc.2016.38. Epub 2016 Mar 7. Oncogene. 2016. PMID: 26947017 Free PMC article.
-
Centriolar satellites are acentriolar assemblies of centrosomal proteins.EMBO J. 2019 Jul 15;38(14):e101082. doi: 10.15252/embj.2018101082. Epub 2019 Jun 3. EMBO J. 2019. PMID: 31304626 Free PMC article.
References
-
- Balestra FR, Strnad P, Fluckiger I, Gonczy P. Discovering regulators of centriole biogenesis through siRNA-based functional genomics in human cells. Dev Cell. 2013;25:555–571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

