Genomic instability in the PARK2 locus is associated with Parkinson's disease
- PMID: 25833766
- PMCID: PMC4617850
- DOI: 10.1007/s13353-015-0282-9
Genomic instability in the PARK2 locus is associated with Parkinson's disease
Abstract
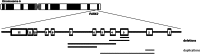
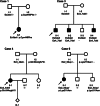
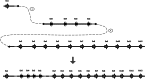
Parkinson's disease (PD) is a common neurodegenerative disorder affecting mostly elderly people, although there is a group of patients developing so-called early-onset PD (EOPD). Mutations in the PARK2 gene are a common cause of autosomal recessive EOPD. PARK2 belongs to the family of extremely large human genes which are often localised in genomic common fragile sites (CFSs) and exhibit gross instability. PARK2 is located in the centre of FRA6E, the third most mutation-susceptible CFS of the human genome. The gene encompasses a region of 1.3 Mbp and, among its mutations, large rearrangements of single or multiple exons account for around 50%. We performed an analysis of the PARK2 gene in a group of 344 PD patients with EOPD and classical form of the disease. Copy number changes were first identified using multiplex ligation probe amplification (MLPA), with their ranges characterised by array comparative genomic hybridisation (aCGH). Exact breakpoints were mapped using direct sequencing. Rearrangements were found in eight subjects, including five deletions and three duplications. Rearrangements were mostly non-recurrent and no repetitive sequences or extended homologies were identified in the regions flanking breakpoint junctions. However, in most cases, 1-3 bp microhomologies were present, strongly suggesting that microhomology-mediated mechanisms, specifically non-homologous end joining (NHEJ) and fork stalling and template switching (FoSTeS)/microhomology-mediated break-induced replication (MMBIR), are predominantly involved in the rearrangement processes in this genomic region.
Keywords: Common fragile sites; FRA6E; Genomic rearrangements; PARK2; Parkinson’s disease.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

