Meiosis and maternal aging: insights from aneuploid oocytes and trisomy births
- PMID: 25833844
- PMCID: PMC4382745
- DOI: 10.1101/cshperspect.a017970
Meiosis and maternal aging: insights from aneuploid oocytes and trisomy births
Abstract
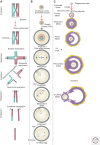
In most organisms, genome haploidization requires reciprocal DNA exchanges (crossovers) between replicated parental homologs to form bivalent chromosomes. These are resolved to their four constituent chromatids during two meiotic divisions. In female mammals, bivalents are formed during fetal life and remain intact until shortly before ovulation. Extending this period beyond ∼35 years greatly increases the risk of aneuploidy in human oocytes, resulting in a dramatic increase in infertility, miscarriage, and birth defects, most notably trisomy 21. Bivalent chromosomes are stabilized by cohesion between sister chromatids, which is mediated by the cohesin complex. In mouse oocytes, cohesin becomes depleted from chromosomes during female aging. Consistent with this, premature loss of centromeric cohesion is a major source of aneuploidy in oocytes from older women. Here, we propose a mechanistic framework to reconcile data from genetic studies on human trisomy and oocytes with recent advances in our understanding of the molecular mechanisms of chromosome segregation during meiosis in model organisms.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Adhikari D, Liu K 2009. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev 30: 438–464. - PubMed
-
- Angell R 1991. Predivision in human oocytes at meiosis I: A mechanism for trisomy formation in man. Hum Genet 86: 383–387. - PubMed
-
- Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH 2008. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol 18: 1514–1519. - PubMed
-
- Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NGM, Verhoeff A, Macklon NS, Fauser BCJM 2007. Milder ovarian stimulation for in vitro fertilization reduces aneuploidy in the human preimplantation embryo: A randomized controlled trial. Hum Reprod 22: 980–988. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
