A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients
- PMID: 25833957
- PMCID: PMC4505010
- DOI: 10.1182/blood-2015-01-623447
A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients
Abstract
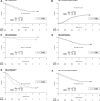
Modifying induction therapy in acute myeloid leukemia (AML) may improve the remission rate and reduce the risk of relapse, thereby improving survival. Escalation of the daunorubicin dose to 90 mg/m(2) has shown benefit for some patient subgroups when compared with a dose of 45 mg/m(2), and has been recommended as a standard of care. However, 60 mg/m(2) is widely used and has never been directly compared with 90 mg/m(2). As part of the UK National Cancer Research Institute (NCRI) AML17 trial, 1206 adults with untreated AML or high-risk myelodysplastic syndrome, mostly younger than 60 years of age, were randomized to a first-induction course of chemotherapy, which delivered either 90 mg/m(2) or 60 mg/m(2) on days 1, 3, and 5 combined with cytosine arabinoside. All patients then received a second course that included daunorubicin 50 mg/m(2) on days 1, 3, and 5. There was no overall difference in complete remission rate (73% vs 75%; odds ratio, 1.07 [0.83-1.39]; P = .6) or in any recognized subgroup. The 60-day mortality was increased in the 90 mg/m(2) arm (10% vs 5% (hazard ratio [HR] 1.98 [1.30-3.02]; P = .001), which resulted in no difference in overall 2-year survival (59% vs 60%; HR, 1.16 [0.95-1.43]; P = .15). In an exploratory subgroup analysis, there was no subgroup that showed significant benefit, although there was a significant interaction by FLT3 ITD mutation. This trial is registered at http://www.isrctn.com as #ISRCTN55675535.
© 2015 by The American Society of Hematology.
Figures

Comment in
-
Beyond the first glance: anthracyclines in AML.Blood. 2015 Jun 18;125(25):3828-9. doi: 10.1182/blood-2015-04-639419. Blood. 2015. PMID: 26089377 No abstract available.
References
-
- Castaigne S, Pautas C, Terré C, et al. Acute Leukemia French Association. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–1516. - PubMed
-
- Récher C, Béné MC, Lioure B, et al. Groupe Ouest-Est d’ étude des Leucé mies Aiguës et autres. Long-term results of a randomized phase 3 trial comparing idarubicin and daunorubicin in younger patients with acute myeloid leukaemia. Leukemia. 2014;28(2):440–443. - PubMed
-
- Burnett AK. Treatment of acute myeloid leukemia: are we making progress? Hematology Am Soc Hematol Educ Program. 2012;2012:1–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

