Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling
- PMID: 25833959
- PMCID: PMC4440883
- DOI: 10.1182/blood-2014-12-618470
Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling
Abstract
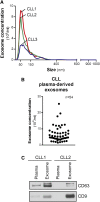
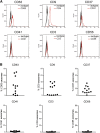
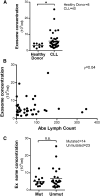
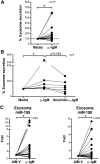
Multiple studies show that chronic lymphocytic leukemia (CLL) cells are heavily dependent on their microenvironment for survival. Communication between CLL cells and the microenvironment is mediated through direct cell contact, soluble factors, and extracellular vesicles. Exosomes are small particles enclosed with lipids, proteins, and small RNAs that can convey biological materials to surrounding cells. Our data herein demonstrate that CLL cells release significant amounts of exosomes in plasma that exhibit abundant CD37, CD9, and CD63 expression. Our work also pinpoints the regulation of B-cell receptor (BCR) signaling in the release of CLL exosomes: BCR activation by α-immunoglobulin (Ig)M induces exosome secretion, whereas BCR inactivation via ibrutinib impedes α-IgM-stimulated exosome release. Moreover, analysis of serial plasma samples collected from CLL patients on an ibrutinib clinical trial revealed that exosome plasma concentration was significantly decreased following ibrutinib therapy. Furthermore, microRNA (miR) profiling of plasma-derived exosomes identified a distinct exosome microRNA signature, including miR-29 family, miR-150, miR-155, and miR-223 that have been associated with CLL disease. Interestingly, expression of exosome miR-150 and miR-155 increases with BCR activation. In all, this study successfully characterized CLL exosomes, demonstrated the control of BCR signaling in the release of CLL exosomes, and uncovered a disease-relevant exosome microRNA profile.
© 2015 by The American Society of Hematology.
Figures


References
-
- Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Semin Cancer Biol. 2014;24:71–81. - PubMed
-
- Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–1847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

