Dietary ω-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin
- PMID: 25833984
- PMCID: PMC4381778
- DOI: 10.3945/ajcn.114.099291
Dietary ω-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin
Abstract
Background: Retinopathy of prematurity (ROP) is a vision-threatening disease in premature infants. Serum adiponectin (APN) concentrations positively correlate with postnatal growth and gestational age, important risk factors for ROP development. Dietary ω-3 (n-3) long-chain polyunsaturated fatty acids (ω-3 LCPUFAs) suppress ROP and oxygen-induced retinopathy (OIR) in a mouse model of human ROP, but the mechanism is not fully understood.
Objective: We examined the role of APN in ROP development and whether circulating APN concentrations are increased by dietary ω-3 LCPUFAs to mediate the protective effect in ROP.
Design: Serum APN concentrations were correlated with ROP development and serum ω-3 LCPUFA concentrations in preterm infants. Mouse OIR was then used to determine whether ω-3 LCPUFA supplementation increases serum APN concentrations, which then suppress retinopathy.
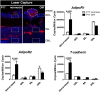
Results: We found that in preterm infants, low serum APN concentrations positively correlate with ROP, and serum APN concentrations positively correlate with serum ω-3 LCPUFA concentrations. In mouse OIR, serum total APN and bioactive high-molecular-weight APN concentrations are increased by ω-3 LCPUFA feed. White adipose tissue, where APN is produced and assembled in the endoplasmic reticulum, is the major source of serum APN. In mouse OIR, adipose endoplasmic reticulum stress is increased, and APN production is suppressed. ω-3 LCPUFA feed in mice increases APN production by reducing adipose endoplasmic reticulum stress markers. Dietary ω-3 LCPUFA suppression of neovascularization is reduced from 70% to 10% with APN deficiency. APN receptors localize in the retina, particularly to pathologic neovessels.
Conclusion: Our findings suggest that increasing APN by ω-3 LCPUFA supplementation in total parental nutrition for preterm infants may suppress ROP.
Keywords: adiponectin; endoplasmic reticulum stress; neovascularization; retinopathy of prematurity; white adipose tissue; ω-3 long-chain polyunsaturated fatty acids.
© 2015 American Society for Nutrition.
Figures






References
-
- Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis 2007;10:133–40. - PubMed
-
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991–9. - PubMed
-
- Palmer EA, Flynn JT, Hardy RJ, Phelps DL, Phillips CL, Schaffer DB, Tung B. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1991;98:1628–40. - PubMed
-
- Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J 2010;425:41–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

