The knockdown of αkap alters the postsynaptic apparatus of neuromuscular junctions in living mice
- PMID: 25834039
- PMCID: PMC4380992
- DOI: 10.1523/JNEUROSCI.3951-14.2015
The knockdown of αkap alters the postsynaptic apparatus of neuromuscular junctions in living mice
Abstract
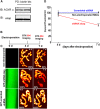
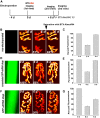
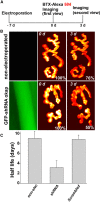
A muscle-specific nonkinase anchoring protein (αkap), encoded within the calcium/calmodulin kinase II (camk2) α gene, was recently found to control the stability of acetylcholine receptor (AChR) clusters on the surface of cultured myotubes. However, it remains unknown whether this protein has any effect on receptor stability and the maintenance of the structural integrity of neuromuscular synapses in vivo. By knocking down the endogenous expression of αkap in mouse sternomastoid muscles with shRNA, we found that the postsynaptic receptor density was dramatically reduced, the turnover rate of receptors at synaptic sites was significantly increased, and the insertion rates of both newly synthesized and recycled receptors into the postsynaptic membrane were depressed. Moreover, we found that αkap shRNA knockdown impaired synaptic structure as postsynaptic AChR clusters and their associated postsynaptic scaffold proteins within the neuromuscular junction were completely eliminated. These results provide new mechanistic insight into the role of αkap in regulating the stability of the postsynaptic apparatus of neuromuscular synapses.
Keywords: NMJ; imaging; knockdown; nAChR dymamics; stability; αkap.
Copyright © 2015 the authors 0270-6474/15/355118-10$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
