Definition of a molecular pathway mediating α-synuclein neurotoxicity
- PMID: 25834048
- PMCID: PMC4380997
- DOI: 10.1523/JNEUROSCI.4650-14.2015
Definition of a molecular pathway mediating α-synuclein neurotoxicity
Erratum in
-
Erratum: Burré et al., "Definition of a Molecular Pathway Mediating α-Synuclein Neurotoxicity".J Neurosci. 2024 May 22;44(21):e0790242024. doi: 10.1523/JNEUROSCI.0790-24.2024. J Neurosci. 2024. PMID: 38744533 Free PMC article. No abstract available.
Abstract
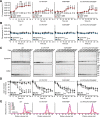
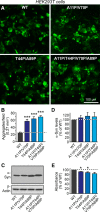
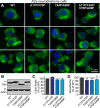
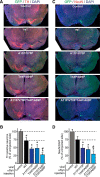
α-Synuclein physiologically chaperones SNARE-complex assembly at the synapse but pathologically misfolds into neurotoxic aggregates that are characteristic for neurodegenerative disorders, such as Parkinson's disease, and that may spread from one neuron to the next throughout the brain during Parkinson's disease pathogenesis. In normal nerve terminals, α-synuclein is present in an equilibrium between a cytosolic form that is natively unfolded and monomeric and a membrane-bound form that is composed of an α-helical multimeric species that chaperones SNARE-complex assembly. Although the neurotoxicity of α-synuclein is well established, the relationship between the native conformations of α-synuclein and its pathological aggregation remain incompletely understood; most importantly, it is unclear whether α-synuclein aggregation originates from its monomeric cytosolic or oligomeric membrane-bound form. Here, we address this question by introducing into α-synuclein point mutations that block membrane binding and by then assessing the effect of blocking membrane binding on α-synuclein aggregation and neurotoxicity. We show that membrane binding inhibits α-synuclein aggregation; conversely, blocking membrane binding enhances α-synuclein aggregation. Stereotactic viral expression of wild-type and mutant α-synuclein in the substantia nigra of mice demonstrated that blocking α-synuclein membrane binding significantly enhanced its neurotoxicity in vivo. Our data delineate a folding pathway for α-synuclein that ranges from a physiological multimeric, α-helical, and membrane-bound species that acts as a SNARE-complex chaperone over a monomeric, natively unfolded form to an amyloid-like aggregate that is neurotoxic in vivo.
Keywords: Parkinson's disease; aggregation; alpha-synuclein; membrane binding.
Copyright © 2015 the authors 0270-6474/15/355221-12$15.00/0.
Figures





References
-
- Azeredo da Silveira S, Schneider BL, Cifuentes-Diaz C, Sage D, Abbas-Terki T, Iwatsubo T, Unser M, Aebischer P. Phosphorylation does not prompt, nor prevent, the formation of alpha-synuclein toxic species in a rat model of Parkinson's disease. Hum Mol Genet. 2009;18:872–887. doi: 10.1093/hmg/ddn417. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
