Contribution of the potassium-chloride cotransporter KCC2 to the strength of inhibition in the neonatal rodent spinal cord in vitro
- PMID: 25834055
- PMCID: PMC6705413
- DOI: 10.1523/JNEUROSCI.1674-14.2015
Contribution of the potassium-chloride cotransporter KCC2 to the strength of inhibition in the neonatal rodent spinal cord in vitro
Abstract
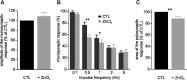
In healthy mature motoneurons (MNs), KCC2 cotransporters maintain the intracellular chloride concentration at low levels, a prerequisite for postsynaptic inhibition mediated by GABA and glycine. KCC2 expression in lumbar MNs is reduced after spinal cord injury (SCI) resulting in a depolarizing shift of the chloride equilibrium potential. Despite modeling studies indicating that such a downregulation of KCC2 function would reduce the strength of postsynaptic inhibition, physiological evidence is still lacking. The present study aimed at investigating the functional impact of a modification of KCC2 function. We focused on a well characterized disynaptic inhibitory pathway responsible for reciprocal inhibition between antagonistic muscles. We performed in vitro extracellular recordings on spinal cords isolated from rodents at the end of the first postnatal week. Genetic reduction of KCC2 expression, pharmacological blockade of KCC2, as well as SCI-induced downregulation of KCC2 all resulted in a reduction of the strength of reciprocal inhibition. We then tried to restore endogenous inhibition after SCI by means of zinc ions that have been shown to boost KCC2 function in other models. Zinc chloride indeed hyperpolarized the chloride equilibrium potential in MNs and increased reciprocal inhibition after neonatal SCI. This study demonstrates that the level of KCC2 function sets the strength of postsynaptic inhibition and suggests that the downregulation of KCC2 after SCI likely contributes to the high occurrence of flexor-extensor cocontractions in SCI patients.
Keywords: KCC2; chloride homeostasis; reciprocal inhibition; spasticity; zinc.
Copyright © 2015 the authors 0270-6474/15/355307-10$15.00/0.
Figures






References
-
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16:302–307. doi: 10.1038/nm.2107. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
