Delayed minimally invasive injection of allogenic bone marrow stromal cell sheets regenerates large bone defects in an ovine preclinical animal model
- PMID: 25834121
- PMCID: PMC4414222
- DOI: 10.5966/sctm.2014-0244
Delayed minimally invasive injection of allogenic bone marrow stromal cell sheets regenerates large bone defects in an ovine preclinical animal model
Abstract
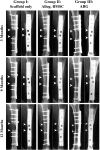
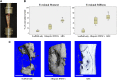
Cell-based tissue engineering approaches are promising strategies in the field of regenerative medicine. However, the mode of cell delivery is still a concern and needs to be significantly improved. Scaffolds and/or matrices loaded with cells are often transplanted into a bone defect immediately after the defect has been created. At this point, the nutrient and oxygen supply is low and the inflammatory cascade is incited, thus creating a highly unfavorable microenvironment for transplanted cells to survive and participate in the regeneration process. We therefore developed a unique treatment concept using the delayed injection of allogenic bone marrow stromal cell (BMSC) sheets to regenerate a critical-sized tibial defect in sheep to study the effect of the cells' regeneration potential when introduced at a postinflammatory stage. Minimally invasive percutaneous injection of allogenic BMSCs into biodegradable composite scaffolds 4 weeks after the defect surgery led to significantly improved bone regeneration compared with preseeded scaffold/cell constructs and scaffold-only groups. Biomechanical testing and microcomputed tomography showed comparable results to the clinical reference standard (i.e., an autologous bone graft). To our knowledge, we are the first to show in a validated preclinical large animal model that delayed allogenic cell transplantation can provide applicable clinical treatment alternatives for challenging bone defects in the future.
Keywords: Allogenic; Bone regeneration; Bone tissue engineering; Cell injection; Large bone defect; Mesenchymal stem cells; Sheep.
©AlphaMed Press.
Figures





References
-
- Xu XQ, Sun W. Perspective from the heart: the potential of human pluripotent stem cell-derived cardiomyocytes. J Cell Biochem. 2013;114:39–46. - PubMed
-
- Vono R, Spinetti G, Gubernator M, et al. What’s new in regenerative medicine: Split up of the mesenchymal stem cell family promises new hope for cardiovascular repair. J Cardiovasc Transl Res. 2012;5:689–699. - PubMed
-
- Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann NY Acad Sci. 2012;1266:107–117. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

