The evolving impact of g protein-coupled receptor kinases in cardiac health and disease
- PMID: 25834229
- PMCID: PMC4551214
- DOI: 10.1152/physrev.00015.2014
The evolving impact of g protein-coupled receptor kinases in cardiac health and disease
Abstract
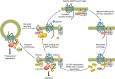
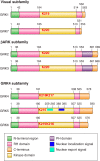
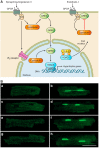
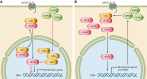
G protein-coupled receptors (GPCRs) are important regulators of various cellular functions via activation of intracellular signaling events. Active GPCR signaling is shut down by GPCR kinases (GRKs) and subsequent β-arrestin-mediated mechanisms including phosphorylation, internalization, and either receptor degradation or resensitization. The seven-member GRK family varies in their structural composition, cellular localization, function, and mechanism of action (see sect. II). Here, we focus our attention on GRKs in particular canonical and novel roles of the GRKs found in the cardiovascular system (see sects. III and IV). Paramount to overall cardiac function is GPCR-mediated signaling provided by the adrenergic system. Overstimulation of the adrenergic system has been highly implicated in various etiologies of cardiovascular disease including hypertension and heart failure. GRKs acting downstream of heightened adrenergic signaling appear to be key players in cardiac homeostasis and disease progression, and herein we review the current data on GRKs related to cardiac disease and discuss their potential in the development of novel therapeutic strategies in cardiac diseases including heart failure.
Copyright © 2015 the American Physiological Society.
Figures

Similar articles
-
G protein-coupled receptor kinases in normal and failing myocardium.Front Biosci (Landmark Ed). 2011 Jun 1;16(8):3047-60. doi: 10.2741/3898. Front Biosci (Landmark Ed). 2011. PMID: 21622221 Free PMC article. Review.
-
G protein-coupled receptor kinases as therapeutic targets in the heart.Nat Rev Cardiol. 2019 Oct;16(10):612-622. doi: 10.1038/s41569-019-0220-3. Epub 2019 Jun 11. Nat Rev Cardiol. 2019. PMID: 31186538 Review.
-
Chapter Three - Ubiquitination and Protein Turnover of G-Protein-Coupled Receptor Kinases in GPCR Signaling and Cellular Regulation.Prog Mol Biol Transl Sci. 2016;141:85-140. doi: 10.1016/bs.pmbts.2016.04.002. Epub 2016 May 7. Prog Mol Biol Transl Sci. 2016. PMID: 27378756 Review.
-
G protein-coupled receptor kinases in hypertension: physiology, pathogenesis, and therapeutic targets.Hypertens Res. 2024 Sep;47(9):2317-2336. doi: 10.1038/s41440-024-01763-y. Epub 2024 Jul 3. Hypertens Res. 2024. PMID: 38961282 Free PMC article. Review.
-
Novel roles for G protein-coupled receptor kinases in cardiac injury and repair.Biochem Soc Trans. 2023 Apr 26;51(2):715-724. doi: 10.1042/BST20221317. Biochem Soc Trans. 2023. PMID: 37013982 Free PMC article. Review.
Cited by
-
The expanding GRK interactome: Implications in cardiovascular disease and potential for therapeutic development.Pharmacol Res. 2016 Aug;110:52-64. doi: 10.1016/j.phrs.2016.05.008. Epub 2016 May 12. Pharmacol Res. 2016. PMID: 27180008 Free PMC article. Review.
-
Antagonistic Roles of GRK2 and GRK5 in Cardiac Aldosterone Signaling Reveal GRK5-Mediated Cardioprotection via Mineralocorticoid Receptor Inhibition.Int J Mol Sci. 2020 Apr 20;21(8):2868. doi: 10.3390/ijms21082868. Int J Mol Sci. 2020. PMID: 32326036 Free PMC article.
-
Impact of Aldosterone on the Failing Myocardium: Insights from Mitochondria and Adrenergic Receptors Signaling and Function.Cells. 2021 Jun 19;10(6):1552. doi: 10.3390/cells10061552. Cells. 2021. PMID: 34205363 Free PMC article. Review.
-
G protein-coupled receptor kinase 5 (GRK5) contributes to impaired cardiac function and immune cell recruitment in post-ischemic heart failure.Cardiovasc Res. 2022 Jan 7;118(1):169-183. doi: 10.1093/cvr/cvab044. Cardiovasc Res. 2022. PMID: 33560342 Free PMC article.
-
Perturbation of the interactions of calmodulin with GRK5 using a natural product chemical probe.Proc Natl Acad Sci U S A. 2019 Aug 6;116(32):15895-15900. doi: 10.1073/pnas.1818547116. Epub 2019 Jul 23. Proc Natl Acad Sci U S A. 2019. PMID: 31337679 Free PMC article.
References
-
- Aguero J, Almenar L, D'Ocon P, Oliver E, Monto F, Moro J, Castello A, Rueda J, Martinez-Dolz L, Sanchez-Lazaro I, Montero JA. Correlation between beta-adrenoceptors and G-protein-coupled receptor kinases in pretransplantation heart failure. Transplant Proc 40: 3014–3016, 2008. - PubMed
-
- Aguero J, Almenar L, D'Ocon P, Oliver E, Monto F, Rueda J, Vicente D, Martinez-Dolz L, Salvador A. Myocardial and peripheral lymphocytic transcriptomic dissociation of beta-adrenoceptors and G protein-coupled receptor kinases in heart transplantation. J Heart Lung Transplant 28: 1166–1171, 2009. - PubMed
-
- Aguero J, Almenar L, Monto F, Oliver E, Sanchez-Lazaro I, Vicente D, Martinez-Dolz L, D'Ocon P, Rueda J, Salvador A. Myocardial G protein receptor-coupled kinase expression correlates with functional parameters and clinical severity in advanced heart failure. J Card Fail 18: 53–61, 2012. - PubMed
-
- Alcantara-Hernandez R, Casas-Gonzalez P, Garcia-Sainz JA. Roles of c-Src in alpha1B-adrenoceptor phosphorylation and desensitization. Auton Autacoid Pharmacol 28: 29–39, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

