Renal autoregulation in health and disease
- PMID: 25834230
- PMCID: PMC4551215
- DOI: 10.1152/physrev.00042.2012
Renal autoregulation in health and disease
Abstract
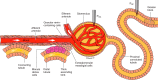
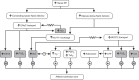
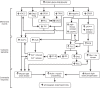
Intrarenal autoregulatory mechanisms maintain renal blood flow (RBF) and glomerular filtration rate (GFR) independent of renal perfusion pressure (RPP) over a defined range (80-180 mmHg). Such autoregulation is mediated largely by the myogenic and the macula densa-tubuloglomerular feedback (MD-TGF) responses that regulate preglomerular vasomotor tone primarily of the afferent arteriole. Differences in response times allow separation of these mechanisms in the time and frequency domains. Mechanotransduction initiating the myogenic response requires a sensing mechanism activated by stretch of vascular smooth muscle cells (VSMCs) and coupled to intracellular signaling pathways eliciting plasma membrane depolarization and a rise in cytosolic free calcium concentration ([Ca(2+)]i). Proposed mechanosensors include epithelial sodium channels (ENaC), integrins, and/or transient receptor potential (TRP) channels. Increased [Ca(2+)]i occurs predominantly by Ca(2+) influx through L-type voltage-operated Ca(2+) channels (VOCC). Increased [Ca(2+)]i activates inositol trisphosphate receptors (IP3R) and ryanodine receptors (RyR) to mobilize Ca(2+) from sarcoplasmic reticular stores. Myogenic vasoconstriction is sustained by increased Ca(2+) sensitivity, mediated by protein kinase C and Rho/Rho-kinase that favors a positive balance between myosin light-chain kinase and phosphatase. Increased RPP activates MD-TGF by transducing a signal of epithelial MD salt reabsorption to adjust afferent arteriolar vasoconstriction. A combination of vascular and tubular mechanisms, novel to the kidney, provides for high autoregulatory efficiency that maintains RBF and GFR, stabilizes sodium excretion, and buffers transmission of RPP to sensitive glomerular capillaries, thereby protecting against hypertensive barotrauma. A unique aspect of the myogenic response in the renal vasculature is modulation of its strength and speed by the MD-TGF and by a connecting tubule glomerular feedback (CT-GF) mechanism. Reactive oxygen species and nitric oxide are modulators of myogenic and MD-TGF mechanisms. Attenuated renal autoregulation contributes to renal damage in many, but not all, models of renal, diabetic, and hypertensive diseases. This review provides a summary of our current knowledge regarding underlying mechanisms enabling renal autoregulation in health and disease and methods used for its study.
Copyright © 2015 the American Physiological Society.
Figures



References
-
- Aalkjaer C, Boedtkjer D, Matchkov V. Vasomotion: what is currently thought? Acta Physiol 202: 253–269, 2011. - PubMed
-
- Abboud HE. Mesangial cell biology. Exp Cell Res 318: 979–985, 2012. - PubMed
-
- Abe Y, Kishimoto T, Yamamoto K. Effect of angiotensin II antagonist infusion on autoregulation of renal blood flow. Am J Physiol 231: 1267–1271, 1976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

