Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs
- PMID: 25834232
- PMCID: PMC4551211
- DOI: 10.1152/physrev.00035.2013
Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs
Abstract
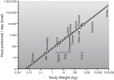
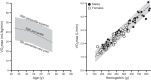
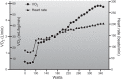
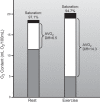
This review focuses on how blood flow to contracting skeletal muscles is regulated during exercise in humans. The idea is that blood flow to the contracting muscles links oxygen in the atmosphere with the contracting muscles where it is consumed. In this context, we take a top down approach and review the basics of oxygen consumption at rest and during exercise in humans, how these values change with training, and the systemic hemodynamic adaptations that support them. We highlight the very high muscle blood flow responses to exercise discovered in the 1980s. We also discuss the vasodilating factors in the contracting muscles responsible for these very high flows. Finally, the competition between demand for blood flow by contracting muscles and maximum systemic cardiac output is discussed as a potential challenge to blood pressure regulation during heavy large muscle mass or whole body exercise in humans. At this time, no one dominant dilator mechanism accounts for exercise hyperemia. Additionally, complex interactions between the sympathetic nervous system and the microcirculation facilitate high levels of systemic oxygen extraction and permit just enough sympathetic control of blood flow to contracting muscles to regulate blood pressure during large muscle mass exercise in humans.
Copyright © 2015 the American Physiological Society.
Figures



References
-
- Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol 59: 1647–1653, 1985. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

