Effect of entacapone on colon motility and ion transport in a rat model of Parkinson's disease
- PMID: 25834315
- PMCID: PMC4375572
- DOI: 10.3748/wjg.v21.i12.3509
Effect of entacapone on colon motility and ion transport in a rat model of Parkinson's disease
Abstract
Aim: To study the effects of entacapone, a catechol-O-methyltransferase inhibitor, on colon motility and electrolyte transport in Parkinson's disease (PD) rats.
Methods: Distribution and expression of catechol-O-methyltransferase (COMT) were measured by immunohistochemistry and Western blotting methods. The colonic smooth muscle motility was examined in vitro by means of a muscle motility recording device. The mucosal electrolyte transport of PD rats was examined by using a short-circuit current (ISC ) technique and scanning ion-selective electrode technique (SIET). Intracellular detection of cAMP and cGMP was accomplished by radioimmunoassay testing.
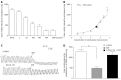
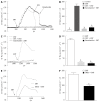
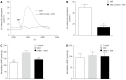
Results: COMT was expressed in the colons of both normal and PD rats, mainly on the apical membranes of villi and crypts in the colon. Compared to normal controls, PD rats expressed less COMT. The COMT inhibitor entacapone inhibited contraction of the PD rat longitudinal muscle in a dose-dependent manner. The β2 adrenoceptor antagonist ICI-118,551 blocked this inhibitory effect by approximately 67% (P < 0.01). Entacapone increased mucosal ISC in the colon of rats with PD. This induction was significantly inhibited by apical application of Cl(-) channel blocker diphenylamine-2, 2'-dicarboxylic acid, basolateral application of Na(+)-K(+)-2Cl(-)co-transporter antagonist bumetanide, elimination of Cl(-) from the extracellular fluid, as well as pretreatment using adenylate cyclase inhibitor MDL12330A. As an inhibitor of prostaglandin synthetase, indomethacin can inhibit entacapone-induced ISC by 45% (P < 0.01). When SIET was applied to measure Cl(-) flux changes, this provided similar results. Entacapone significantly increased intracellular cAMP content in the colonic mucosa, which was greatly inhibited by indomethacin.
Conclusion: COMT expression exists in rat colons. The β2 adrenoceptor is involved in the entacapone-induced inhibition of colon motility. Entacapone induces cAMP-dependent Cl(-) secretion in the PD rat.
Keywords: Catechol-O-methyltransferase; Colon motility; Entacapone; Ion transport; Parkinson disease.
Figures


Similar articles
-
Entacapone promotes cAMP-dependent colonic Cl(-) secretion in rats.Neurogastroenterol Motil. 2011 Jul;23(7):657-e277. doi: 10.1111/j.1365-2982.2011.01715.x. Epub 2011 Apr 19. Neurogastroenterol Motil. 2011. PMID: 21501335
-
Entacapone, a catechol-O-methyltransferase inhibitor, improves the motor activity and dopamine content of basal ganglia in a rat model of Parkinson's disease induced by Japanese encephalitis virus.Brain Res. 2010 Jan 14;1309:110-5. doi: 10.1016/j.brainres.2009.10.055. Epub 2009 Oct 29. Brain Res. 2010. PMID: 19879254
-
Entacapone: a catechol-O-methyltransferase inhibitor for the adjunctive treatment of Parkinson's disease.Clin Ther. 2001 Jun;23(6):802-32; discussion 771. doi: 10.1016/s0149-2918(01)80071-0. Clin Ther. 2001. PMID: 11440283 Review.
-
Traditional Chinese formula, lubricating gut pill, improves loperamide-induced rat constipation involved in enhance of Cl- secretion across distal colonic epithelium.J Ethnopharmacol. 2010 Jul 20;130(2):347-53. doi: 10.1016/j.jep.2010.05.018. Epub 2010 May 19. J Ethnopharmacol. 2010. PMID: 20488235
-
[Inhibition of the COMPT with entacapone in the treatment of motor fluctuations in Parkinson disease].Neurologia. 1999 Aug-Sep;14(7):349-58. Neurologia. 1999. PMID: 10570622 Review. Spanish.
Cited by
-
Entacapone alleviates muscle atrophy by modulating oxidative stress, proteolysis, and lipid aggregation in multiple mice models.Front Physiol. 2024 Dec 9;15:1483594. doi: 10.3389/fphys.2024.1483594. eCollection 2024. Front Physiol. 2024. PMID: 39717825 Free PMC article.
-
Colonic Fluid and Electrolyte Transport 2022: An Update.Cells. 2022 May 22;11(10):1712. doi: 10.3390/cells11101712. Cells. 2022. PMID: 35626748 Free PMC article. Review.
-
Putative regulation of macrophage-mediated inflammation by catestatin.Trends Immunol. 2022 Jan;43(1):41-50. doi: 10.1016/j.it.2021.11.002. Epub 2021 Nov 27. Trends Immunol. 2022. PMID: 34844850 Free PMC article. Review.
-
Non-invasive micro-test technology and applications.Biophys Rep. 2025 Apr 30;11(2):96-111. doi: 10.52601/bpr.2024.240009. Biophys Rep. 2025. PMID: 40308937 Free PMC article.
References
-
- Rajput AH, Uitti RJ, Rajput A, Offord KP. Mortality in Parkinson’s disease. Mov Disord. 2010;25:507–508. - PubMed
-
- Carecchio M, Collini A, Comi C, Cantello R, Bhatia KP, Monaco F. Levodopa-induced belly dancer’s dyskinesias in Parkinson’s disease: report of one case. Mov Disord. 2010;25:1760–1762. - PubMed
-
- Tambasco N, Belcastro V, Gallina A, Castrioto A, Calabresi P, Rossi A. Levodopa-induced breathing, cognitive and behavioral changes in Parkinson’s disease. J Neurol. 2011;258:2296–2299. - PubMed
-
- Kurtis MM, Franch O. Tardive syndrome and Parkinson’s disease responsive to concomitant tetrabenazine and levodopa therapy. Parkinsonism Relat Disord. 2011;17:774–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

