Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK- and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells
- PMID: 25834400
- PMCID: PMC4365758
- DOI: 10.2147/DDDT.S76057
Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK- and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells
Abstract
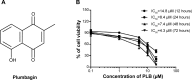
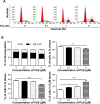
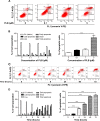
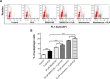
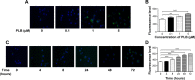
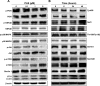
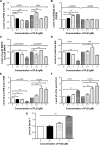
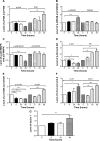
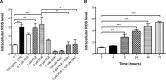
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone; PLB), a naturally occurring naphthoquinone isolated from the roots of Plumbaginaceae plants, has been reported to possess anticancer activities in both in vitro and in vivo studies, but the effect of PLB on tongue squamous cell carcinoma (TSCC) is not fully understood. This study aimed to investigate the effects of PLB on cell cycle distribution, apoptosis, and autophagy, and the underlying mechanisms in the human TSCC cell line SCC25. The results have revealed that PLB exerted potent inducing effects on cell cycle arrest, apoptosis, and autophagy in SCC25 cells. PLB arrested SCC25 cells at the G2/M phase in a concentration- and time-dependent manner with a decrease in the expression level of cell division cycle protein 2 homolog (Cdc2) and cyclin B1 and increase in the expression level of p21 Waf1/Cip1, p27 Kip1, and p53 in SCC25 cells. PLB markedly induced apoptosis and autophagy in SCC25 cells. PLB decreased the expression of the anti-apoptotic proteins B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-xl) while increasing the expression level of the pro-apoptotic protein Bcl-2-associated X protein (Bax) in SCC25 cells. Furthermore, PLB inhibited phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR), glycogen synthase kinase 3β (GSK3β), and p38 mitogen-activated protein kinase (p38 MAPK) pathways as indicated by the alteration in the ratio of phosphorylation level over total protein expression level, contributing to the autophagy inducing effect. In addition, we found that wortmannin (a PI3K inhibitor) and SB202190 (a selective inhibitor of p38 MAPK) strikingly enhanced PLB-induced autophagy in SCC25 cells, suggesting the involvement of PI3K- and p38 MAPK-mediated signaling pathways. Moreover, PLB induced intracellular reactive oxygen species (ROS) generation and this effect was attenuated by l-glutathione (GSH) and n-acetyl-l-cysteine (NAC). Taken together, these results indicate that PLB promotes cellular apoptosis and autophagy in TSCC cells involving p38 MAPK- and PI3K/Akt/mTOR-mediated pathways with contribution from the GSK3β and ROS-mediated pathways.
Keywords: GSK3β; ROS; TSCC; cell cycle; p38 MAPK.
Figures








References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. - PubMed
-
- Dogan E, Cetinayak HO, Sarioglu S, Erdag TK, Ikiz AO. Patterns of cervical lymph node metastases in oral tongue squamous cell carcinoma: implications for elective and therapeutic neck dissection. J Laryngol Otol. 2014;128(3):268–273. - PubMed
-
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368(19):1845–1846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

