Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures
- PMID: 25834433
- PMCID: PMC4370919
- DOI: 10.2147/IJN.S75615
Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures
Abstract
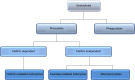
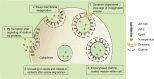
Cellular internalization and trans-barrier transport of nanoparticles can be manipulated on the basis of the physicochemical and mechanical characteristics of nanoparticles. Research has shown that these factors significantly influence the uptake of nanoparticles. Dictating these characteristics allows for the control of the rate and extent of cellular uptake, as well as delivering the drug-loaded nanosystem intra-cellularly, which is imperative for drugs that require a specific cellular level to exert their effects. Additionally, physicochemical characteristics of the nanoparticles should be optimal for the nanosystem to bypass the natural restricting phenomena of the body and act therapeutically at the targeted site. The factors at the focal point of emerging smart nanomedicines include nanoparticle size, surface charge, shape, hydrophobicity, surface chemistry, and even protein and ligand conjugates. Hence, this review discusses the mechanism of internalization of nanoparticles and ideal nanoparticle characteristics that allow them to evade the biological barriers in order to achieve optimal cellular uptake in different organ systems. Identifying these parameters assists with the progression of nanomedicine as an outstanding vector of pharmaceuticals.
Keywords: cellular uptake; charge; nanoparticles; shape; size; transport mechanisms.
Figures






Similar articles
-
Intracellular uptake, transport, and processing of gold nanostructures.Mol Membr Biol. 2010 Oct;27(7):299-311. doi: 10.3109/09687688.2010.507787. Epub 2010 Oct 7. Mol Membr Biol. 2010. PMID: 20929337 Review.
-
Promoting Inter-/Intra- Cellular Process of Nanomedicine through its Physicochemical Properties Optimization.Curr Drug Metab. 2018;19(1):75-82. doi: 10.2174/1389200219666171221122119. Curr Drug Metab. 2018. PMID: 29268683 Review.
-
Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles.J Control Release. 2009 May 5;135(3):259-67. doi: 10.1016/j.jconrel.2009.01.018. Epub 2009 Feb 3. J Control Release. 2009. PMID: 19331853
-
Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking.Small. 2013 May 27;9(9-10):1521-32. doi: 10.1002/smll.201201390. Epub 2012 Sep 28. Small. 2013. PMID: 23019091 Review.
-
Exploiting endocytosis for nanomedicines.Cold Spring Harb Perspect Biol. 2013 Nov 1;5(11):a016980. doi: 10.1101/cshperspect.a016980. Cold Spring Harb Perspect Biol. 2013. PMID: 24186069 Free PMC article. Review.
Cited by
-
Enhanced in Vitro Anti-Tumor Activity of 5-Azacytidine by Entrapment into Solid Lipid Nanoparticles.Adv Pharm Bull. 2016 Sep;6(3):367-375. doi: 10.15171/apb.2016.048. Epub 2016 Sep 25. Adv Pharm Bull. 2016. PMID: 27766220 Free PMC article.
-
Nanotechnology-Based Cardiac Targeting and Direct Cardiac Reprogramming: The Betrothed.Stem Cells Int. 2017;2017:4940397. doi: 10.1155/2017/4940397. Epub 2017 Dec 11. Stem Cells Int. 2017. PMID: 29375623 Free PMC article. Review.
-
Design of Cyclic Peptide-Based Nanospheres and the Delivery of siRNA.Int J Mol Sci. 2022 Oct 11;23(20):12071. doi: 10.3390/ijms232012071. Int J Mol Sci. 2022. PMID: 36292932 Free PMC article.
-
Mitochondria-Targeted Lipid Nanoparticles Loaded with Rotenone as a New Approach for the Treatment of Oncological Diseases.Molecules. 2023 Oct 23;28(20):7229. doi: 10.3390/molecules28207229. Molecules. 2023. PMID: 37894708 Free PMC article.
-
NK Cells in the Tumor Microenvironment as New Potential Players Mediating Chemotherapy Effects in Metastatic Melanoma.Front Oncol. 2021 Oct 12;11:754541. doi: 10.3389/fonc.2021.754541. eCollection 2021. Front Oncol. 2021. PMID: 34712615 Free PMC article. Review.
References
-
- Alonso MJ. Nanomedicines for overcoming biological barriers. Biomed Pharmacother. 2004;58(3):168–172. - PubMed
-
- Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8(2):147–166. - PubMed
-
- Brzoska M, Langer K, Coester C, Loitsch S, Wagner TO, Mallinckrodt C. Incorporation of biodegradable nanoparticles into human airway epithelium cells-in vitro study of the suitability as a vehicle for drug or gene delivery in pulmonary diseases. Biochem Biophys Res Commun. 2004;318(2):562–570. - PubMed
-
- Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

