Survey on appropriateness of use of nimesulide in nine European countries
- PMID: 25834467
- PMCID: PMC4365741
- DOI: 10.2147/DHPS.S76320
Survey on appropriateness of use of nimesulide in nine European countries
Abstract
Background: Appropriateness of use is a key factor in safeguarding patient's health as well as a product's therapeutic properties. This paper presents the results of a survey conducted in nine European countries to verify the appropriateness of use of nimesulide in patients with inflammatory pain.
Methods: Computer-aided telephone interviews were administered to 1,277 nimesulide-prescribing general practitioners in Bulgaria, Czech Republic, Greece, Hungary, Italy, Poland, Portugal, Romania, and Slovakia, covering an estimated 31,719 patients. The interview questionnaire collected information on nimesulide prescriptions with respect to daily dose, treatment duration, and indication.
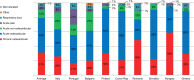
Results: In the majority of cases, prescriptions of nimesulide did not exceed the recommended daily dose of 200 mg (given as 100 mg twice a day), with a range from 161 mg (Greece) to 190 mg (Slovakia). An adherence to the 15-day treatment limit was observed in over 90% of cases. The average number of treatment days was always less than 15, with a range from 5.4 (Italy) to 13.6 (Czech Republic). Nimesulide was primarily used for the treatment of acute pain and short-term painful episodes in chronic conditions. The presence of gastrointestinal diseases/ulcers was the most frequent reason for not prescribing nimesulide.
Conclusion: The results of this survey demonstrate that nimesulide is generally prescribed in compliance with the information reported in the summary of product characteristics (SmPC) with regard to daily dose and treatment duration, and suggest that it is mainly used for the management of episodes of acute pain in patients with a chronic disorder. These findings indicate the appropriateness of use of nimesulide in the European countries considered in this survey.
Keywords: European Medicine Agency; NSAID; appropriateness of use; computer-aided telephone interviews; non-steroidal anti-inflammatory drug.
Figures


Similar articles
-
Nimesulide: patients still exposed to a risk of severe hepatitis.Prescrire Int. 2011 May;20(116):125-6. Prescrire Int. 2011. PMID: 21648177
-
Treatment of acute low back pain with the COX-2-selective anti-inflammatory drug nimesulide: results of a randomized, double-blind comparative trial versus ibuprofen.Spine (Phila Pa 1976). 2000 Jun 15;25(12):1579-85. doi: 10.1097/00007632-200006150-00019. Spine (Phila Pa 1976). 2000. PMID: 10851109 Clinical Trial.
-
[Nimesulide: 25 years later].Minerva Med. 2010 Aug;101(4):285-93. Minerva Med. 2010. PMID: 21030939 Review. Italian.
-
Pattern of NSAID use in the Italian general population: a questionnaire-based survey.Eur J Clin Pharmacol. 2004 Dec;60(10):731-8. doi: 10.1007/s00228-004-0826-0. Epub 2004 Oct 26. Eur J Clin Pharmacol. 2004. PMID: 15517225
-
Nimesulide. An update of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy.Drugs. 1994 Sep;48(3):431-54. doi: 10.2165/00003495-199448030-00009. Drugs. 1994. PMID: 7527762 Review.
Cited by
-
Mixed Impact of Direct Healthcare Professional Communications When Considering Proximal Outcomes and the Targeted Population: A Systematic Review.Pharmacoepidemiol Drug Saf. 2025 Mar;34(3):e70135. doi: 10.1002/pds.70135. Pharmacoepidemiol Drug Saf. 2025. PMID: 40122533 Free PMC article.
-
A survey and optical microscopy in pilot comparative analysis of generic and original nimesulide granules.Heliyon. 2021 Jul 6;7(7):e07490. doi: 10.1016/j.heliyon.2021.e07490. eCollection 2021 Jul. Heliyon. 2021. PMID: 34345723 Free PMC article.
References
-
- European Medicines Agency Summary of Product Characteristics. Annex III to the Commision Decision on Article 31 referral for nimesulide-containing medicinal products. [Accessed July 28, 2014]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referr.... Updated January 20, 2012.
-
- European Medicines Agency Nimesulide – referrals. [Accessed July 28, 2014]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referr.... Updated April 19, 2012.
-
- European Medicines Agency Assessment report for Nimesulide containing medicinal products-Procedure number: EMEA/H/A-31/1261- 20 January 2012 – Report Nr.EMA/73856/2012 -Patient Health Protection. [Accessed July 28, 2014]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/....
-
- Ketola E, Klockars M. Computer-assisted telephone interview (CATI) in primary care. Fam Pract. 1999;16:179–183. - PubMed
-
- Anie KA, Jones PW, Hilton SR, Anderson HR. A computer-assisted telephone interview technique for assessment of asthma morbidity and drug use in adult asthma. J Clin Epidemiol. 1996;49:653–656. - PubMed
LinkOut - more resources
Full Text Sources

