Perinatal asphyxia leads to PARP-1 overactivity, p65 translocation, IL-1β and TNF-α overexpression, and apoptotic-like cell death in mesencephalon of neonatal rats: prevention by systemic neonatal nicotinamide administration
- PMID: 25835215
- PMCID: PMC4383817
- DOI: 10.1007/s12640-015-9517-0
Perinatal asphyxia leads to PARP-1 overactivity, p65 translocation, IL-1β and TNF-α overexpression, and apoptotic-like cell death in mesencephalon of neonatal rats: prevention by systemic neonatal nicotinamide administration
Abstract
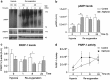
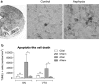
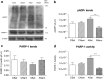
Perinatal asphyxia (PA) is a leading cause of neuronal damage in newborns, resulting in long-term neurological and cognitive deficits, in part due to impairment of mesostriatal and mesolimbic neurocircuitries. The insult can be as severe as to menace the integrity of the genome, triggering the overactivation of sentinel proteins, including poly (ADP-ribose) polymerase-1 (PARP-1). PARP-1 overactivation implies increased energy demands, worsening the metabolic failure and depleting further NAD(+) availability. Using a global PA rat model, we report here evidence that hypoxia increases PARP-1 activity, triggering a signalling cascade leading to nuclear translocation of the NF-κB subunit p65, modulating the expression of IL-1β and TNF-α, pro-inflammatory molecules, increasing apoptotic-like cell death in mesencephalon of neonate rats, monitored with Western blots, qPCR, TUNEL and ELISA. PARP-1 activity increased immediately after PA, reaching a maximum 1-8 h after the insult, while activation of the NF-κB signalling pathway was observed 8 h after the insult, with a >twofold increase of p65 nuclear translocation. IL-1β and TNF-α mRNA levels were increased 24 h after the insult, together with a >twofold increase in apoptotic-like cell death. A single dose of the PARP-1 inhibitor nicotinamide (0.8 mmol/kg, i.p.), 1 h post delivery, prevented the effect of PA on PARP-1 activity, p65 translocation, pro-inflammatory cytokine expression and apoptotic-like cell death. The present study demonstrates that PA leads to PARP-1 overactivation, increasing the expression of pro-inflammatory cytokines and cell death in mesencephalon, effects prevented by systemic neonatal nicotinamide administration, supporting the idea that PARP-1 inhibition represents a therapeutic target against the effects of PA.
Figures


Similar articles
-
Targeting Sentinel Proteins and Extrasynaptic Glutamate Receptors: a Therapeutic Strategy for Preventing the Effects Elicited by Perinatal Asphyxia?Neurotox Res. 2018 Feb;33(2):461-473. doi: 10.1007/s12640-017-9795-9. Epub 2017 Aug 26. Neurotox Res. 2018. PMID: 28844085 Free PMC article. Review.
-
Further studies on the hypothesis of PARP-1 inhibition as a strategy for lessening the long-term effects produced by perinatal asphyxia: effects of nicotinamide and theophylline on PARP-1 activity in brain and peripheral tissue : nicotinamide and theophylline on PARP-1 activity.Neurotox Res. 2012 Jul;22(1):79-90. doi: 10.1007/s12640-012-9310-2. Epub 2012 Feb 4. Neurotox Res. 2012. PMID: 22311271
-
Short- and long-term consequences of perinatal asphyxia: looking for neuroprotective strategies.Adv Neurobiol. 2015;10:169-98. doi: 10.1007/978-1-4939-1372-5_9. Adv Neurobiol. 2015. PMID: 25287541
-
Poly(ADP-ribose) polymerase 1 inhibition prevents interleukin-1β-induced inflammation in human osteoarthritic chondrocytes.Acta Biochim Biophys Sin (Shanghai). 2015 Jun;47(6):422-30. doi: 10.1093/abbs/gmv033. Epub 2015 Apr 29. Acta Biochim Biophys Sin (Shanghai). 2015. PMID: 25926140
-
Perinatal asphyxia: CNS development and deficits with delayed onset.Front Neurosci. 2014 Mar 26;8:47. doi: 10.3389/fnins.2014.00047. eCollection 2014. Front Neurosci. 2014. PMID: 24723845 Free PMC article. Review.
Cited by
-
Gold nanorods/siRNA complex administration for knockdown of PARP-1: a potential treatment for perinatal asphyxia.Int J Nanomedicine. 2018 Oct 25;13:6839-6854. doi: 10.2147/IJN.S175076. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30498346 Free PMC article.
-
A Controversial Medicolegal Issue: Timing the Onset of Perinatal Hypoxic-Ischemic Brain Injury.Mediators Inflamm. 2017;2017:6024959. doi: 10.1155/2017/6024959. Epub 2017 Aug 13. Mediators Inflamm. 2017. PMID: 28883688 Free PMC article. Review.
-
Targeting Sentinel Proteins and Extrasynaptic Glutamate Receptors: a Therapeutic Strategy for Preventing the Effects Elicited by Perinatal Asphyxia?Neurotox Res. 2018 Feb;33(2):461-473. doi: 10.1007/s12640-017-9795-9. Epub 2017 Aug 26. Neurotox Res. 2018. PMID: 28844085 Free PMC article. Review.
-
Effects of Poly(ADP-Ribose) Polymerase-1 Inhibition in a Neonatal Rodent Model of Hypoxic-Ischemic Injury.Biomed Res Int. 2017;2017:2924848. doi: 10.1155/2017/2924848. Epub 2017 Jun 15. Biomed Res Int. 2017. PMID: 28698869 Free PMC article.
-
Sustained Energy Deficit Following Perinatal Asphyxia: A Shift towards the Fructose-2,6-bisphosphatase (TIGAR)-Dependent Pentose Phosphate Pathway and Postnatal Development.Antioxidants (Basel). 2021 Dec 29;11(1):74. doi: 10.3390/antiox11010074. Antioxidants (Basel). 2021. PMID: 35052577 Free PMC article. Review.
References
-
- Allende-Castro C, Espina-Marchant P, Bustamante D, Rojas-Mancilla E, Neira T, Gutierrez-Hernandez MA, Esmar D, Valdes JL, Morales P, Gebicke-Haerter PJ, Herrera-Marschitz M. Further studies on the hypothesis of PARP-1 inhibition as strategy for lessening the long-term effects produced by perinatal asphyxia: effects of nicotinamide and theophylline on PARP-1 activity in brain and peripheral tissue. Neurotox Res. 2012;22:79–90. doi: 10.1007/s12640-012-9310-2. - DOI - PubMed
-
- Basovich SN. The role of hypoxia in mental development and in the treatment of mental disorders. BioSci Trends. 2010;4:288–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

