Viable neuronopathic Gaucher disease model in Medaka (Oryzias latipes) displays axonal accumulation of alpha-synuclein
- PMID: 25835295
- PMCID: PMC4383526
- DOI: 10.1371/journal.pgen.1005065
Viable neuronopathic Gaucher disease model in Medaka (Oryzias latipes) displays axonal accumulation of alpha-synuclein
Abstract
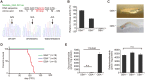
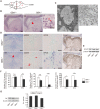
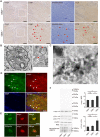
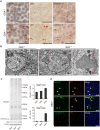
Homozygous mutations in the glucocerebrosidase (GBA) gene result in Gaucher disease (GD), the most common lysosomal storage disease. Recent genetic studies have revealed that GBA mutations confer a strong risk for sporadic Parkinson's disease (PD). To investigate how GBA mutations cause PD, we generated GBA nonsense mutant (GBA-/-) medaka that are completely deficient in glucocerebrosidase (GCase) activity. In contrast to the perinatal death in humans and mice lacking GCase activity, GBA-/- medaka survived for months, enabling analysis of the pathological progression. GBA-/- medaka displayed the pathological phenotypes resembling human neuronopathic GD including infiltration of Gaucher cell-like cells into the brains, progressive neuronal loss, and microgliosis. Detailed pathological findings represented lysosomal abnormalities in neurons and alpha-synuclein (α-syn) accumulation in axonal swellings containing autophagosomes. Unexpectedly, disruption of α-syn did not improve the life span, formation of axonal swellings, neuronal loss, or neuroinflammation in GBA-/- medaka. Taken together, the present study revealed GBA-/- medaka as a novel neuronopathic GD model, the pahological mechanisms of α-syn accumulation caused by GCase deficiency, and the minimal contribution of α-syn to the pathogenesis of neuronopathic GD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Impact of Gba2 on neuronopathic Gaucher's disease and α-synuclein accumulation in medaka (Oryzias latipes).Mol Brain. 2021 May 10;14(1):80. doi: 10.1186/s13041-021-00790-x. Mol Brain. 2021. PMID: 33971917 Free PMC article.
-
Viral delivery of a microRNA to Gba to the mouse central nervous system models neuronopathic Gaucher disease.Neurobiol Dis. 2019 Oct;130:104513. doi: 10.1016/j.nbd.2019.104513. Epub 2019 Jun 21. Neurobiol Dis. 2019. PMID: 31233883
-
Gaucher-Associated Parkinsonism.Cell Mol Neurobiol. 2015 Aug;35(6):755-61. doi: 10.1007/s10571-015-0176-8. Epub 2015 Mar 29. Cell Mol Neurobiol. 2015. PMID: 25820783 Free PMC article. Review.
-
Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a Gaucher mutation.Brain. 2014 Dec;137(Pt 12):3235-47. doi: 10.1093/brain/awu291. Epub 2014 Oct 27. Brain. 2014. PMID: 25351739 Free PMC article.
-
[GBA mutations and Parkinson's disease].Sheng Li Xue Bao. 2018 Jun 25;70(3):294-300. Sheng Li Xue Bao. 2018. PMID: 29926071 Review. Chinese.
Cited by
-
In vivo Magnetic Resonance Microscopy and Hypothermic Anaesthesia of a Disease Model in Medaka.Sci Rep. 2016 Jun 2;6:27188. doi: 10.1038/srep27188. Sci Rep. 2016. PMID: 27251889 Free PMC article.
-
Parkinson's disease pathogenesis from the viewpoint of small fish models.J Neural Transm (Vienna). 2018 Jan;125(1):25-33. doi: 10.1007/s00702-017-1772-1. Epub 2017 Aug 2. J Neural Transm (Vienna). 2018. PMID: 28770388 Review.
-
Current trends in basic research on Parkinson's disease: from mitochondria, lysosome to α-synuclein.J Neural Transm (Vienna). 2024 Jun;131(6):663-674. doi: 10.1007/s00702-024-02774-2. Epub 2024 Apr 13. J Neural Transm (Vienna). 2024. PMID: 38613675 Free PMC article. Review.
-
Intact in vivo visualization of telencephalic microvasculature in medaka using optical coherence tomography.Sci Rep. 2020 Nov 16;10(1):19831. doi: 10.1038/s41598-020-76468-6. Sci Rep. 2020. PMID: 33199719 Free PMC article.
-
Impact of Gba2 on neuronopathic Gaucher's disease and α-synuclein accumulation in medaka (Oryzias latipes).Mol Brain. 2021 May 10;14(1):80. doi: 10.1186/s13041-021-00790-x. Mol Brain. 2021. PMID: 33971917 Free PMC article.
References
-
- Futerman AH, Zimran A (2006) Gaucher disease: CRC Press.
-
- Wong K, Sidransky E, Verma A, Mixon T, Sandberg GD, et al. (2004) Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab 82: 192–207. - PubMed
-
- Eblan MJ, Goker-Alpan O, Sidransky E (2005) Perinatal lethal Gaucher disease: a distinct phenotype along the neuronopathic continuum. Fetal Pediatr Pathol 24: 205–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

