Profiles of influenza A/H1N1 vaccine response using hemagglutination-inhibition titers
- PMID: 25835513
- PMCID: PMC4514374
- DOI: 10.1080/21645515.2015.1011990
Profiles of influenza A/H1N1 vaccine response using hemagglutination-inhibition titers
Abstract
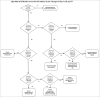
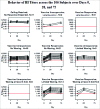
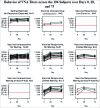
To identify distinct antibody profiles among adults 50-to-74 years old using influenza A/H1N1 HI titers up to 75 days after vaccination. Healthy subjects 50 to 74 years old received the 2010-2011 trivalent inactivated influenza vaccine. We measured venous samples from Days 0, 28, and 75 for HI and VNA and B-cell ELISPOTs. Of 106 subjects, HI titers demonstrated a ceiling effect for 11 or 10% for those with a pre-vaccination HI titer of 1:640 where no subject post-vaccination had an increase in titer. Of the remaining 95 subjects, only 37 or 35% overall had at least a 4-fold increase by Day 28. Of these 37, 3 waned at least 4-fold, and 13 others 2-fold. Thus 15% of the subjects showed waning antibody titers by Day 75. More than half failed to respond at all. The profiles populated by these subjects as defined by HI did not vary with age or gender. The VNA results mimicked the HI profiles, but the profiles for B-cell ELISPOT did not. HI titers at Days 0, 28, and 75 populate 4 biologically plausible profiles. Limitations include lack of consensus for operationally defining waning as well as for the apparent ceiling. Furthermore, though well accepted as a marker for vaccine response, assigning thresholds with HI has limitations. However, VNA closely matches HI in populating these profiles. Thus, we hold that these profiles, having face- and content-validity, may provide a basis for understanding variation in genomic and transcriptomic response to influenza vaccination in this age group.
Keywords: ASC, Antibody-Secreting Cells; ELISPOT, Enzyme-Linked ImmunoSpot; Et al., Et alia (and others); H1N1 subtype; HI, Hemagglutination-Inhibition; IQR, Interquartile Range; IgG, Immunoglobulin G; MDCK, Madin-Darby Canine Kidney; PFU, Plaque-Forming Units; RBC, Red Blood Cells; TCID50, Tissue Culture Infectious Dose 50; VNA, Virus Neutralization Assay; WHO, World Health Organization; aging; antibodies; hemagglutination inhibition tests; hemagglutinin glycoproteins; influenza a virus; influenza vaccines; influenza virus; p, p-value; viral; μl, Microliters.
Figures
Similar articles
-
Pre-vaccination immunity and immune responses to a cell culture-derived whole-virus H1N1 vaccine are similar to a seasonal influenza vaccine.Vaccine. 2012 Jun 22;30(30):4543-51. doi: 10.1016/j.vaccine.2012.03.061. Epub 2012 Apr 1. Vaccine. 2012. PMID: 22475864 Clinical Trial.
-
Pandemic influenza A H1N1 vaccine in recipients of solid organ transplants: immunogenicity and tolerability outcomes after vero cell derived, non-adjuvanted, whole-virion vaccination.Vaccine. 2011 Sep 16;29(40):6888-93. doi: 10.1016/j.vaccine.2011.07.050. Epub 2011 Jul 29. Vaccine. 2011. PMID: 21803100
-
Poor immune response to a standard single dose non-adjuvanted vaccination against 2009 pandemic H1N1 influenza virus A in the adult and elder hemodialysis patients.Vaccine. 2012 Jul 13;30(33):5009-18. doi: 10.1016/j.vaccine.2012.05.016. Epub 2012 May 30. Vaccine. 2012. PMID: 22658967
-
Safety and immunogenicity in man of a cell culture derived trivalent live attenuated seasonal influenza vaccine: a Phase I dose escalating study in healthy volunteers.Vaccine. 2014 Sep 3;32(39):5118-24. doi: 10.1016/j.vaccine.2014.05.030. Epub 2014 May 23. Vaccine. 2014. PMID: 24858566 Clinical Trial.
-
Host immune response to A(H1N1)pdm09 vaccination and infection: a one-year prospective study on six cohorts of subjects.Vaccine. 2012 Jul 6;30(32):4785-9. doi: 10.1016/j.vaccine.2012.05.030. Epub 2012 May 24. Vaccine. 2012. PMID: 22633868
Cited by
-
COVID-19 vaccines and beyond.Cell Mol Immunol. 2024 Mar;21(3):207-209. doi: 10.1038/s41423-024-01132-2. Epub 2024 Jan 26. Cell Mol Immunol. 2024. PMID: 38273150 Free PMC article. No abstract available.
-
Characterizing the Short- and Long-Term Temporal Dynamics of Antibody Responses to Influenza Vaccination.medRxiv [Preprint]. 2025 Feb 27:2025.02.26.25322965. doi: 10.1101/2025.02.26.25322965. medRxiv. 2025. PMID: 40061340 Free PMC article. Preprint.
-
Statistical modeling using early markers of innate immunity to explain variation in humoral responses to influenza vaccine in older adults.Vaccine. 2015 Jul 17;33(31):3682-8. doi: 10.1016/j.vaccine.2015.06.031. Epub 2015 Jun 16. Vaccine. 2015. PMID: 26087295 Free PMC article.
-
Variation by lineage in serum antibody responses to influenza B virus infections.PLoS One. 2020 Nov 9;15(11):e0241693. doi: 10.1371/journal.pone.0241693. eCollection 2020. PLoS One. 2020. PMID: 33166348 Free PMC article.
-
Sex Differences in Older Adults' Immune Responses to Seasonal Influenza Vaccination.Front Immunol. 2019 Feb 27;10:180. doi: 10.3389/fimmu.2019.00180. eCollection 2019. Front Immunol. 2019. PMID: 30873150 Free PMC article.
References
-
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol 2001; 75:12182-7; PMID:11711609; http://dx.doi.org/10.1128/JVI.75.24.12182-12187.2001 - DOI - PMC - PubMed
-
- Bernstein E, Kaye D, Abrutyn E, Gross P, Dorfman M, Murasko DM. Immune response to influenza vaccination in a large healthy elderly population. Vaccine 1999; 17:82-94; PMID:10078611; http://dx.doi.org/10.1016/S0264-410X(98)00117-0 - DOI - PubMed
-
- Nicholls S, Carroll K, Crofts J, Ben-Eliezer E, Paul J, Zambon M, Joseph CA, Verlander NQ, Goddard NL, Watson JM. Outbreak of influenza A (H3N2) in a highly-vaccinated religious community: a retrospective cohort study. Commun Dis Public Health 2004; 7:272-7; PMID:15779788 - PubMed
-
- Engler RJ, Nelson MR, Klote MM, VanRaden MJ, Huang CY, Cox NJ, Klimov A, Keitel WA, Nichol KL, Carr WW, et al. . Half- vs full-dose trivalent inactivated influenza vaccine (2004-2005): age, dose, and sex effects on immune responses. Arch Intern Med 2008; 168:2405-14; PMID:19064822; http://dx.doi.org/10.1001/archinternmed.2008.513 - DOI - PubMed
-
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490-502; PMID:18275271; http://dx.doi.org/10.1086/524146 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous