Impaired T cell responsiveness to interleukin-6 in hematological patients with invasive aspergillosis
- PMID: 25835547
- PMCID: PMC4383538
- DOI: 10.1371/journal.pone.0123171
Impaired T cell responsiveness to interleukin-6 in hematological patients with invasive aspergillosis
Abstract
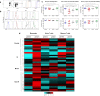
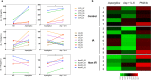
Invasive mold infections (IMI) are among the most devastating complications following chemotherapy and hematopoietic stem cell transplantation (HSCT), with high mortality rates. Yet, the molecular basis for human susceptibility to invasive aspergillosis (IA) and mucormycosis remain poorly understood. Herein, we aimed to characterize the immune profile of individuals with hematological malignancies (n = 18) who developed IMI during the course of chemotherapy or HSCT, and compared it to that of hematological patients who had no evidence of invasive fungal infection (n = 16). First, we measured the expression of the pattern recognition receptors pentraxin 3, dectin-1, and Toll-like receptors (TLR) 2 and 4 in peripheral blood of chemotherapy and HSCT recipients with IMI. Compared to hematological controls, individuals with IA and mucormycosis had defective expression of dectin-1; in addition, patients with mucormycosis had decreased TLR2 and increased TLR4 expression. Since fungal recognition via dectin-1 favors T helper 17 responses and the latter are highly dependent on activation of the signal transducer and activator of transcription (STAT) 3, we next used phospho-flow cytometry to measure the phosphorylation of the transcription factors STAT1 and STAT3 in response to interferon-gamma (IFN-γ) and interleukin (IL)-6, respectively. While IFN-γ/STAT1 signaling was similar between groups, naïve T cells from patients with IA, but not those with mucormycosis, exhibited reduced responsiveness to IL-6 as measured by STAT3 phosphorylation. Furthermore, IL-6 increased Aspergillus-induced IL-17 production in culture supernatants from healthy and hematological controls but not in patients with IA. Altogether, these observations suggest an important role for dectin-1 and the IL-6/STAT3 pathway in protective immunity against Aspergillus.
Conflict of interest statement
Figures

References
-
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010; 50(8):1091–1100. 10.1086/651263 - DOI - PubMed
-
- Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 2009; 48(3):265–273. 10.1086/595846 - DOI - PubMed
-
- Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100(13):4358–4366. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

