Mitochondrial vulnerability and increased susceptibility to nutrient-induced cytotoxicity in fibroblasts from leigh syndrome French canadian patients
- PMID: 25835550
- PMCID: PMC4383560
- DOI: 10.1371/journal.pone.0120767
Mitochondrial vulnerability and increased susceptibility to nutrient-induced cytotoxicity in fibroblasts from leigh syndrome French canadian patients
Abstract
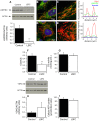
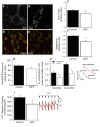
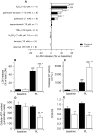
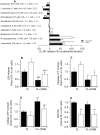
Mutations in LRPPRC are responsible for the French Canadian variant of Leigh Syndrome (LSFC), a severe disorder characterized biochemically by a tissue-specific deficiency of cytochrome c oxidase (COX) and clinically by the occurrence of severe and deadly acidotic crises. Factors that precipitate these crises remain unclear. To better understand the physiopathology and identify potential treatments, we performed a comprehensive analysis of mitochondrial function in LSFC and control fibroblasts. Furthermore, we have used this cell-based model to screen for conditions that promote premature cell death in LSFC cells and test the protective effect of ten interventions targeting well-defined aspects of mitochondrial function. We show that, despite maintaining normal ATP levels, LSFC fibroblasts present several mitochondrial functional abnormalities under normal baseline conditions, which likely impair their capacity to respond to stress. This includes mitochondrial network fragmentation, impaired oxidative phosphorylation capacity, lower membrane potential, increased sensitivity to Ca2+-induced permeability transition, but no changes in reactive oxygen species production. We also show that LSFC fibroblasts display enhanced susceptibility to cell death when exposed to palmitate, an effect that is potentiated by high lactate, while high glucose or acidosis alone or in combination were neutral. Furthermore, we demonstrate that compounds that are known to promote flux through the electron transport chain independent of phosphorylation (methylene blue, dinitrophenol), or modulate fatty acid (L-carnitine) or Krebs cycle metabolism (propionate) are protective, while antioxidants (idebenone, N-acetyl cysteine, resveratrol) exacerbate palmitate plus lactate-induced cell death. Collectively, beyond highlighting multiple alterations in mitochondrial function and increased susceptibility to nutrient-induced cytotoxicity in LSFC fibroblasts, these results raise questions about the nature of the diets, particularly excess fat intake, as well as on the use of antioxidants in patients with LSFC and, possibly, other COX defects.
Conflict of interest statement
Figures
References
-
- Xu F, Morin C, Mitchell G, Ackerley C, Robinson BH (2004) The role of the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III mRNA. Biochem J 382: 331 10.1042/BJ20040469 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

