Vagus nerve stimulation for partial seizures
- PMID: 25835947
- PMCID: PMC7138043
- DOI: 10.1002/14651858.CD002896.pub2
Vagus nerve stimulation for partial seizures
Update in
-
Vagus nerve stimulation for focal seizures.Cochrane Database Syst Rev. 2022 Jul 14;7(7):CD002896. doi: 10.1002/14651858.CD002896.pub3. Cochrane Database Syst Rev. 2022. PMID: 35833911 Free PMC article.
Abstract
Background: Vagus nerve stimulation (VNS) is a neuromodulatory treatment that is used as an adjunctive therapy for treating people with medically refractory epilepsy. VNS consists of chronic intermittent electrical stimulation of the vagus nerve, delivered by a programmable pulse generator. The majority of people given a diagnosis of epilepsy have a good prognosis, and their seizures will be controlled by treatment with a single antiepileptic drug (AED), but up to 20%-30% of patients will develop drug-resistant epilepsy, often requiring treatment with combinations of AEDs. The aim of this systematic review was to overview the current evidence for the efficacy and tolerability of vagus nerve stimulation when used as an adjunctive treatment for people with drug-resistant partial epilepsy. This is an updated version of a Cochrane review published in Issue 7, 2010.
Objectives: To determine:(1) The effects on seizures of VNS compared to controls e.g. high-level stimulation compared to low-level stimulation (presumed sub-therapeutic dose); and(2) The adverse effect profile of VNS compared to controls e.g. high-level stimulation compared to low-level stimulation.
Search methods: We searched the Cochrane Epilepsy Group's Specialised Register (23 February 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 23 February 2015), MEDLINE (1946 to 23 February 2015), SCOPUS (1823 to 23 February 2015), ClinicalTrials.gov (23 February 2015) and ICTRP (23 February 2015). No language restrictions were imposed.
Selection criteria: The following study designs were eligible for inclusion: randomised, double-blind, parallel or crossover studies, controlled trials of VNS as add-on treatment comparing high and low stimulation paradigms (including three different stimulation paradigms - duty cycle: rapid, mid and slow) and VNS stimulation versus no stimulation or a different intervention. Eligible participants were adults or children with drug-resistant partial seizures not eligible for surgery or who failed surgery.
Data collection and analysis: Two review authors independently selected trials for inclusion and extracted data. The following outcomes were assessed: (a) 50% or greater reduction in total seizure frequency; (b) treatment withdrawal (any reason); (c) adverse effects; (d) quality of life; (e) cognition; (f) mood. Primary analyses were intention-to-treat. Sensitivity best and worst case analyses were also undertaken to account for missing outcome data. Pooled Risk Ratios (RR) with 95% confidence intervals (95% Cl) were estimated for the primary outcomes of seizure frequency and treatment withdrawal. For adverse effects, pooled RRs and 99% CI's were calculated.
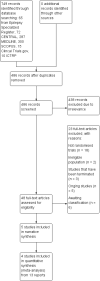
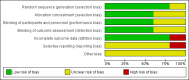
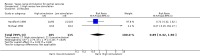
Main results: Five trials recruited a total of 439 participants and between them compared different types of VNS stimulation therapy. Baseline phase ranged from 4 to 12 weeks and double-blind treatment phases from 12 to 20 weeks in the five trials. Overall, two studies were rated as having a low risk of bias and three had an unclear risk of bias due to lack of reported information around study design. Effective blinding of studies of VNS is difficult due to the frequency of stimulation-related side effects such as voice alteration; this may limit the validity of the observed treatment effects. Four trials compared high frequency stimulation to low frequency stimulation and were included in quantitative syntheses (meta-analyses).The overall risk ratio (95% CI) for 50% or greater reduction in seizure frequency across all studies was 1.73 (1.13 to 2.64) showing that high frequency VNS was over one and a half times more effective than low frequency VNS. For this outcome, we rated the evidence as being moderate in quality due to incomplete outcome data in one included study; however results did not vary substantially and remained statistically significant for both the best and worst case scenarios. The risk ratio (RR) for treatment withdrawal was 2.56 (0.51 to 12.71), however evidence for this outcome was rated as low quality due to imprecision of the result and incomplete outcome data in one included study. The RR of adverse effects were as follows: (a) voice alteration and hoarseness 2.17 (99% CI 1.49 to 3.17); (b) cough 1.09 (99% CI 0.74 to 1.62); (c) dyspnea 2.45 (99% CI 1.07 to 5.60); (d) pain 1.01 (99% CI 0.60 to 1.68); (e) paresthesia 0.78 (99% CI 0.39 to 1.53); (f) nausea 0.89 (99% CI 0.42 to 1.90); (g) headache 0.90 (99% CI 0.48 to 1.69); evidence of adverse effects was rated as moderate to low quality due to imprecision of the result and/or incomplete outcome data in one included study. No important heterogeneity between studies was found for any of the outcomes.
Authors' conclusions: VNS for partial seizures appears to be an effective and well tolerated treatment in 439 included participants from five trials. Results of the overall efficacy analysis show that VNS stimulation using the high stimulation paradigm was significantly better than low stimulation in reducing frequency of seizures. Results for the outcome "withdrawal of allocated treatment" suggest that VNS is well tolerated as withdrawals were rare. No significant difference was found in withdrawal rates between the high and low stimulation groups, however limited information was available from the evidence included in this review so important differences between high and low stimulation cannot be excluded . Adverse effects associated with implantation and stimulation were primarily hoarseness, cough, dyspnea, pain, paresthesia, nausea and headache, with hoarseness and dyspnea more likely to occur on high stimulation than low stimulation. However, the evidence on these outcomes is limited and of moderate to low quality. Further high quality research is needed to fully evaluate the efficacy and tolerability of VNS for drug resistant partial seizures.
Conflict of interest statement
Nothing to declare.
Figures











Update of
-
Vagus nerve stimulation for partial seizures.Cochrane Database Syst Rev. 2002;(1):CD002896. doi: 10.1002/14651858.CD002896. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2015 Apr 03;(4):CD002896. doi: 10.1002/14651858.CD002896.pub2. PMID: 11869641 Updated.
References
References to studies included in this review
DeGiorgio 2005 {published data only}
-
- DeGiorgio C, Heck C, Bunch S, Britton J, Green P, Lancman M, et al. Vagus nerve stimulation for epilepsy: randomized comparison of three stimulation paradigms. Neurology 2005;65(2):317‐9. - PubMed
Handforth 1998 {published data only}
-
- Amar AP, Heck C, Levi M, Smith T, DeGiorgio CM, Oviedo S, et al. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique and outcome. Neurosurgery 1998;43(6):1265‐76. - PubMed
-
- Dodrill CB, Morris GL. Effects of vagal nerve stimulation on cognition and quality of life in epilepsy. Epilepsy & Behavior 2000;2(1):46‐53. - PubMed
-
- Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial‐onset seizures: a randomized active‐control trial. Neurology 1998;51(1):48‐55. - PubMed
Klinkenberg 2012 {published data only}
-
- Aalbers MW, Klinkenberg S, Rijkers K, Verschuure P, Kessels A, Aldenkamp A, et al. The effects of vagus nerve stimulation on pro‐ and anti‐inflammatory cytokines in children with refractory epilepsy: an exploratory study. Neuroimmunomodulation 2012;19(6):352‐8. - PubMed
-
- Klinkenberg S, Aalbers MW, Vles JSH, Cornips EMJ, Rijkers K, Leenen L, et al. Vagus nerve stimulation in children with intractable epilepsy: a randomized controlled trial. Development Medicine & Child Neurology 2012;54(9):855‐61. - PubMed
Michael 1993 {published data only}
-
- Michael JE, Wegener K, Barners DW. Vagus nerve stimulation for intractable seizures: one year follow‐up. Journal of Neuroscience Nursing 1993;25(6):362‐6. - PubMed
VNS Study Group 1995 {published data only}
-
- Ben‐Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Research 1995;20(3):221‐7. - PubMed
-
- Ben‐Menachem E, Manon‐Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia 1994;35(3):616‐26. - PubMed
-
- Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Research 2000;42(2‐3):203‐10. - PubMed
-
- Holder LK, Wernicke JF, Tarver WB. Treatment of refractory partial seizures: preliminary results of a controlled study. Pacing & Clinical Electrophysiology 1992;15(10 Pt 2):1557‐71. - PubMed
-
- Lotvall J, Lunde H, Augustinson LE, Hedner T, Svedmyr N, Ben‐Menachem E. Airway effects of direct left‐sided cervical vagal stimulation in patients with complex partial seizures. Epilepsy Research 1994;18(2):149‐54. - PubMed
References to studies excluded from this review
Clarke 1997 {published data only}
-
- Clarke BM, Upton ARM, Griffin H, Fitzpatrick D, DeNardis M. Seizure control after stimulation of the vagus nerve: clinical outcome measures. Canadian Journal of Neurological Sciences 1997;24(3):222‐5. - PubMed
Cukiert 2013 {published data only}
-
- Cukiert A, Cukiert CM, Burattini JA, LIma AM, Forster CR, Baise C, et al. A prospective long‐term study on the outcome after vagus nerve stimulation at maximally tolerated current intensity in a cohort of children with refractory secondary generalized epilepsy. Neuromodulation 2013;16(6):551‐6. [DOI: 10.1111/j.1525-1403.2012.00522.x; PUBMED: 23738578 ] - DOI - PubMed
DeGiorgio 2000 {published data only}
-
- DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, et al. Prospective long term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia 2000;41(9):1195‐2000. - PubMed
Garcia‐Navarrete 2013 {published data only}
-
- Garcia‐Navarrete E, Torres CV, Gallego I, Navas M, Pastor J, Sola RG. Long‐term results of vagal nerve stimulation for adults with medication‐resistant epilepsy who have been on unchanged antiepileptic medication. Seizure 2013;22(1):9‐13. - PubMed
George 1994 {published data only}
-
- George R, Salinsky M, Kuzniecky R, Rosenfeld W, Bergen D, Tarver WB, et al. Vagus nerve stimulation for treatment of partial seizures: 3. Long‐term follow‐up on first 67 patients exiting a controlled study. Epilepsia 1994;35(3):637‐43. - PubMed
Hornig 1997 {published data only}
-
- Hornig GW, Murphy JV, Schallert G, Tilton C. Left vagus nerve stimulation in children with refractory epilepsy: an update. Southern Medical Journal 1997;90(5):484‐8. - PubMed
Marras 2013 {published data only}
-
- Marras CE, Chiesa V, Benedictis A, Franzini A, Rizzi M, Villani F, et al. Vagus nerve stimulation in refractory epilepsy: new indications and outcome assessment. Epilepsy and Behavior 2013;28(3):374‐8. - PubMed
Marrosu 2003 {published data only}
-
- Marrosu F, Serra A, Maleci A, Puligheddu M, Biggio G, Piga M. Correlation between GABA‐A receptor density and vagus nerve stimulation in individuals with drug‐resistant partial epilepsy. Epilepsy Research 2003;55(1‐2):59‐70. - PubMed
Marrosu 2005 {published data only}
-
- Marrosu F, Santoni F, Puligheddu M, Barberini L, Maleci A, Ennas F, et al. Increase in 20‐50 Hz (gamma frequencies) power spectrum and synchronization after chronic vagal nerve stimulation. Clinical Neurophysiology 2005;116(9):2026‐36. - PubMed
Morris 1999 {published data only}
-
- Morris GL 3rd, Mueller WM, The Vagus Nerve Stimulation Group. Long‐term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurology 1999;53(8):1731‐5. - PubMed
Muller 2010 {published data only}
-
- Muller K, Fabo D, Entz L, Kelemen A, Halasz P, Rasonyi G, et al. Outcome of vagus nerve stimulation for epilepsy in Budapest. Epilepsia 2010;51(Suppl.3):98‐101. - PubMed
NCT00215215 2005 {published data only}
-
- NCT00215215. Effectiveness study comparing treatment with drug(s) or adjunctive VNS therapy for pharmacoresistant partial seizures. http://ClinicalTrials.gov/show/NCT00215215 2005.
NCT01118455 2004 {published data only}
-
- NCT01118455. Trial to assess vagus nerve stimulation therapy vs. anti‐epileptic drug treatment in children with refractory seizures. http://ClinicalTrials.gov/show/NCT01118455 2004.
NCT01178437 2009 {published data only}
-
- NCT01178437. Transcutaneous non‐invasive stimulation of the vagus nerve for the treatment of difficult‐to‐treat epilepsy. http://ClinicalTrials.gov/show/NCT01178437 2009.
Ronkainem 2006 {published data only}
-
- Ronkainen E, Korpelainen JT, Heikkinen E, Myllyla VV, Huikuri HV, Isojarvi JIT. Cardiac autonomic control in patients with refractory epilepsy before and during vagus nerve stimulation treatment: a one‐year follow‐up study. Epilepsia 2006;47(3):556‐562. - PubMed
Rossignol 2009 {published data only}
-
- Rossignol E, Lortie A, Thomas T, Bouthiller A, Scavarda D, Mercier C, et al. Vagus nerve stimulation in pediatric epileptic syndromes. Seizure 2009;18(1):34‐7. - PubMed
Salinsky 1996 {published data only}
-
- Salinsky MC, Uthman BM, Ristanovic RK, Wernicke JF, Tarver WB, The Vagus Nerve Stimulation Study Group. Vagus nerve stimulation for the treatment of medically intractable seizures: results of a 1‐year open‐extension trial. Archives of Neurology 1996;53(11):1176‐80. - PubMed
Scherrrmann 2001 {published data only}
-
- Scherrmann J, Hoppe C, Kral T, Schramm J, Elger CE. Vagus nerve stimulation: clinical experience in a large patient series. Journal of Clinical Neurophysiology 2001;18(5):408‐14. - PubMed
Shimizu 1995 {published data only}
-
- Shimizu H, Ishijima B, Nakamura K, Asakura T, Ohtsuki T, Yoshimoto T, et al. Effect of vagal stimulation on intractable epilepsy. Psychiatry and Clinical Neurosciences 1995;49(3):S254‐5. - PubMed
Sirven 2000 {published data only}
-
- Sirven JI, Sperling M, Naritoku D, Schachter S, Labar D, Holmes M, et al. Vagus nerve stimulation therapy for epilepsy in older adults. Neurology 2000;54(3):1179‐1182. - PubMed
Uthman 1993 {published data only}
-
- Uthman BM, Wilder BJ, Penry JK, Dean C, Ramsay RE, Reid SA, et al. Treatment of epilepsy by stimulation of the vagus nerve. Neurology 1993;43(7):1338‐45. - PubMed
Wang 2004 {published data only}
-
- Wang LN, Zhao LF, Wang YZ. Basic and clinical study of vagus nerve stimulation in the treatment of epilepsy. Chinese Journal of Clinical Rehabilitation 2004;8(13):2516‐7.
Wang 2009 {published data only}
-
- Wang H, Chen X, Lin Z, Shao Z, Sun B, Shen H, et al. Long‐term effect of vagus nerve stimulation on interictal epileptiform discharges in refractory epilepsy. Journal of the Neurological Sciences 2009;284(1‐2):96‐102. - PubMed
References to studies awaiting assessment
Boon 2001 {published data only}
-
- Boon P, Vonck K, D'Havé M, Laere K, Michielsen G, Thiery E, et al. Results of vagus nerve stimulation for refractory epilepsy in Ghent, Belgium. Abstracts of the XVII World Congress of Neurology. 2001.
Gates 2005 {published data only}
-
- Gates JR, Liow K, Granner M, Louis E, Leroy R, Albers L, et al. Vagus nerve stimulator (VNS) parameter settings: high stimulation vs low stimulation [abstract]. Epilepsia 2005;46(Suppl 8):223, Abstract no: 2.390.
Klinkenberg 2014 {published data only}
-
- Klinkenberg S, Borne CJH, Aalbers MW, Verschuure P, Kessels AG, et al. The effects of vagus nerve stimulation on tryptophan metabolites in children with intractable epilepsy. Epilepsy & behavior 2014;37:133‐8. - PubMed
Landy 1993 {published data only}
-
- Landy HJ, Ramsay RE, Slater J, Casiano RR, Morgan R. Vagus nerve stimulation for complex partial seizures: surgical technique, safety, and efficacy. Journal of Neurosurgery 1993;78(1):26‐31. - PubMed
Leach 2001 {published data only}
-
- Leach JP, Leach VM, Harrison E, Chadwick DW, Eldridge P. Vagal nerve stimulation: a blinded controlled trial of efficacy [abstract]. Epilepsia 2001;42(Suppl 7):206, Abstract no: 2.334.
Ney 1998 {published data only}
-
- Ney G. Vagus nerve stimulation. American Journal of Managed Care 1998;4(9):S495‐500.
Scherrmann 2001 {published data only}
-
- Scherrmann JM, Hoppe C, Kuczaty S, Sassen R, Elger CE. Vagus nerve stimulation (VNS): efficacy of standard stimulation versus rapid cycle: a randomized controlled trial [abstract]. Epilepsia 2001;42(Suppl 7):282, Abstract no: 3.205.
References to ongoing studies
DRKS00003689 2012 {published data only}
-
- DRKS00003689. A randomized, controlled, double‐blind, two‐arm clinical trial to assess safety and efficacy of transcutaneous vagus nerve stimulation (t‐VNS®) in patients with drug‐resistant epilepsy. http://www.drks.de/DRKS00003689 2012.
NCT00782249 2005 {published data only}
-
- NCT00782249. Trial comparing different stimulation paradigms in patients treated with vagus nerve stimulation for refractory epilepsy. http://ClinicalTrials.gov/show/NCT00782249 2005.
NCT01281293 2011 {published data only}
-
- NCT01281293. Vagus nerve stimulation clinical outcomes measured prospectively in patients stimulated. http://clinicaltrials.gov/show/NCT01281293 2011.
NCT01378611 2006 {published data only}
-
- NCT01378611. Does VNS interact with the serotonergic and immune system in children with intractable epilepsy?. http://ClinicalTrials.gov/show/NCT01378611 2006.
NCT01910129 2013 {published data only}
-
- NCT01910129. Neurostimulation to the vagus nerve for the reduction in frequency of seizures associated with epilepsy. http://ClinicalTrials.gov/show/NCT01910129 2013.
NCT02089243 2014 {published data only}
-
- NCT02089243. Controlled Randomized Vagus Nerve Stimulation (VNS) Therapy Versus Resection (CoRaVNStiR). http://ClinicalTrials.gov/show/NCT02089243 2014.
Additional references
Cockerell 1995
-
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet 1995;346(8979):140‐4. - PubMed
Forsgren 2005
-
- Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe ‐ a systematic review. European Journal of Neurology 2005;12(4):245‐53. - PubMed
Hallbook 2005
-
- Hallbook T, Lundgren J, Stjernqvist K, Blennow G, Strömblad L‐G, Rosén I. Vagus nerve stimulation in 15 children with therapy resistant epilepsy; its impact on cognition, quality of life, behaviour and mood. Seizure 2005;14(7):504‐513. - PubMed
Hauser 1975
-
- Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia 1975;16(1):1‐66. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kirkham 2010
-
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: ] - PubMed
Krahl 2012
Kwan 2000
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New England Journal of Medicine 2000;342(5):314‐9. - PubMed
Morris 2013
Sackeim 2001
-
- Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, et al. Vagus Nerve Stimulation (VNS) for treatment‐resistant depression: efficacy, side effects and predictors of outcome. Neuropsychopharmacology 2001;25(5):713–28. - PubMed
Schachter 1998
-
- Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia 1998;39(7):677‐86. - PubMed
Selway 1987
-
- Selway R, Nashef L. Vagus Nerve Stimulation. From Membranes to Mankind‐ A Practical Guide to Epilepsy. Vol. 48, Epilepsy Society, 1987.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

