Tumor Budding Correlates With the Protumor Immune Microenvironment and Is an Independent Prognostic Factor for Recurrence of Stage I Lung Adenocarcinoma
- PMID: 25836013
- PMCID: PMC4556124
- DOI: 10.1378/chest.14-3005
Tumor Budding Correlates With the Protumor Immune Microenvironment and Is an Independent Prognostic Factor for Recurrence of Stage I Lung Adenocarcinoma
Abstract
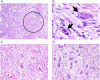
Background: Immune cell infiltration associated with tumor capsule disruption and tumor budding has been shown to reflect invasiveness, metastasis, and unfavorable prognosis in colorectal cancer. We investigated the influence of tumor budding on prognosis and its association with the immune microenvironment in lung adenocarcinoma.
Methods: Tumor slides from resected stage I lung adenocarcinomas were reviewed (n = 524 and n = 514, for training and validation cohorts, respectively) for assessment of tumor budding. CD3+ and forkhead box P3+ (FoxP3+) lymphocytes, CD68+ macrophages, IL-7 receptor, and IL-12 receptor β2 were analyzed using tissue microarrays constructed from tumor and stroma. Probability of recurrence was calculated using the competing risks method.
Results: In the training cohort, risk of recurrence for high-grade tumor budding was higher than it was for low-grade tumor budding (32% vs 12%, P < .001), which was confirmed in the validation cohort (P = .005). Tumor budding stratified the risk of recurrence for acinar-predominant (22% vs 9%, P < .001), papillary-predominant (22% vs 13%, P = .045), and solid-predominant (39% vs 19%, P = .022) tumors. Tumor budding was associated with higher stromal FoxP3+ lymphocyte infiltration, higher stromal FoxP3/CD3 risk index, higher tumoral and stromal CD68+ macrophage infiltration, and IL-7 receptor overexpression (P < .001, all associations). Tumor budding remained independently associated with recurrence on multivariate analysis (hazard ratio, 1.61; P = .008).
Conclusions: Tumor budding is an independent prognostic factor of stage I lung adenocarcinoma and correlates with the protumor immune microenvironment. Our findings advocate investigating tumor-immune cell interactions at the invading edge as a biologic driver of tumor aggressiveness.
Figures




References
-
- Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117(2):294-299. - PubMed
-
- van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer. 2007;120(4):868-874. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

