BRCA1 and BRCA2 protect against oxidative DNA damage converted into double-strand breaks during DNA replication
- PMID: 25836596
- PMCID: PMC4442488
- DOI: 10.1016/j.dnarep.2015.03.002
BRCA1 and BRCA2 protect against oxidative DNA damage converted into double-strand breaks during DNA replication
Abstract
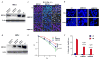
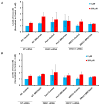
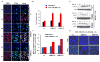
BRCA1 and BRCA2 mutation carriers are predisposed to develop breast and ovarian cancers, but the reasons for this tissue specificity are unknown. Breast epithelial cells are known to contain elevated levels of oxidative DNA damage, triggered by hormonally driven growth and its effect on cell metabolism. BRCA1- or BRCA2-deficient cells were found to be more sensitive to oxidative stress, modeled by treatment with patho-physiologic concentrations of hydrogen peroxide. Hydrogen peroxide exposure leads to oxidative DNA damage induced DNA double strand breaks (DSB) in BRCA-deficient cells causing them to accumulate in S-phase. In addition, after hydrogen peroxide treatment, BRCA deficient cells showed impaired Rad51 foci which are dependent on an intact BRCA1-BRCA2 pathway. These DSB resulted in an increase in chromatid-type aberrations, which are characteristic for BRCA1 and BRCA2-deficient cells. The most common result of oxidative DNA damage induced processing of S-phase DSB is an interstitial chromatid deletion, but insertions and exchanges were also seen in BRCA deficient cells. Thus, BRCA1 and BRCA2 are essential for the repair of oxidative DNA damage repair intermediates that persist into S-phase and produce DSB. The implication is that oxidative stress plays a role in the etiology of hereditary breast cancer.
Keywords: BRCA; Cancer; Chromosome aberrations; Homologous recombination; Oxidative stress.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
Simon Powell have no financial conflicts of interest with any of the work in this submitted paper.
Simon Powell have a single position of employment (Memorial Sloan-Kettering) and no other sources of income.
Figures


References
-
- McCord JM. Superoxide dismutase in aging and disease: an overview. Methods Enzymol. 2002;349:331–41. - PubMed
-
- Hursting SD, et al. Mechanism-based cancer prevention approaches: targets, examples, and the use of transgenic mice. J Natl Cancer Inst. 1999;91(3):215–25. - PubMed
-
- Lavigne JA, et al. The effects of catechol-O-methyltransferase inhibition on estrogen metabolite and oxidative DNA damage levels in estradiol-treated MCF-7 cells. Cancer Res. 2001;61(20):7488–94. - PubMed
-
- Hamada J, et al. Increased oxidative DNA damage in mammary tumor cells by continuous epidermal growth factor stimulation. J Natl Cancer Inst. 2001;93(3):214–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

