Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings
- PMID: 25836634
- PMCID: PMC4576934
- DOI: 10.1021/ja513079r
Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings
Abstract
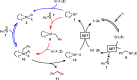
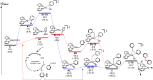
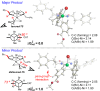
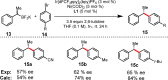
The cross-coupling of sp(3)-hybridized organoboron reagents via photoredox/nickel dual catalysis represents a new paradigm of reactivity for engaging alkylmetallic reagents in transition-metal-catalyzed processes. Reported here is an investigation into the mechanistic details of this important transformation using density functional theory. Calculations bring to light a new reaction pathway involving an alkylnickel(I) complex generated by addition of an alkyl radical to Ni(0) that is likely to operate simultaneously with the previously proposed mechanism. Analysis of the enantioselective variant of the transformation reveals an unexpected manifold for stereoinduction involving dynamic kinetic resolution (DKR) of a Ni(III) intermediate wherein the stereodetermining step is reductive elimination. Furthermore, calculations suggest that the DKR-based stereoinduction manifold may be responsible for stereoselectivity observed in numerous other stereoconvergent Ni-catalyzed cross-couplings and reductive couplings.
Figures
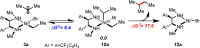
References
-
- Metal-Catalyzed Cross-Coupling Reactions; de Meijere A., Diederich F., Eds.; Wiley-VCH: Weinheim, Germany, 2004.
-
- Hartwig J. F.Organotransition Metal Chemistry: From Bonding to Catalysis, 3rd ed.; University Science: Sausalito, CA, 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

