Reprogramming Mouse Cells With a Pancreatic Duct Phenotype to Insulin-Producing β-Like Cells
- PMID: 25836667
- PMCID: PMC4430605
- DOI: 10.1210/en.2014-1987
Reprogramming Mouse Cells With a Pancreatic Duct Phenotype to Insulin-Producing β-Like Cells
Abstract
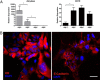
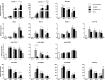
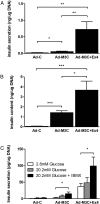
Reprogramming technology has opened the possibility of converting one cell type into another by forced expression of transgenes. Transduction of adenoviral vectors encoding 3 pancreatic transcription factors, Pdx1, Ngn3, and MafA, into mouse pancreas results in direct reprogramming of exocrine cells to insulin-producing β-like cells. We hypothesized that cultured adult pancreatic duct cells could be reprogrammed to become insulin-producing β-cells by adenoviral-mediated expression of this same combination of factors. Exocrine were isolated from adult mouse insulin 1 promoter (MIP)-green fluorescent protein (GFP) transgenic mice to allow new insulin-expressing cells to be detected by GFP fluorescence. Cultured cells were transduced by an adenoviral vector carrying a polycistronic construct Ngn3/Pdx1/MafA/mCherry (Ad-M3C) or mCherry sequence alone as a control vector. In addition, the effects of glucagon-like peptide-1 (GLP-1) receptor agonist, exendin-4 (Ex-4) on the reprogramming process were examined. GFP(+) cells appeared 2 days after Ad-M3C transduction; the reprogramming efficiency was 8.6 ± 2.6% by day 4 after transduction. Ad-M3C also resulted in increased expression of β-cell markers insulin 1 and 2, with enhancement by Ex-4. Expression of other β-cell markers, neuroD and GLP-1 receptor, were also significantly up-regulated. The amount of insulin release into the media and insulin content of the cells were significantly higher in the Ad-M3C-transduced cells; this too was enhanced by Ex-4. The transduced cells did not secrete insulin in response to increased glucose, indicating incomplete differentiation to β-cells. Thus, cultured murine adult pancreatic cells with a duct phenotype can be directly reprogrammed to insulin-producing β-like cells by adenoviral delivery of 3 pancreatic transcription factors.
Figures


References
-
- Alejandro R, Barton FB, Hering BJ, Wease S. 2008 update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86(12):1783–1788. - PubMed
-
- Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

