A randomised trial of lung sealant versus medical therapy for advanced emphysema
- PMID: 25837041
- PMCID: PMC4826269
- DOI: 10.1183/09031936.00205614
A randomised trial of lung sealant versus medical therapy for advanced emphysema
Abstract
Uncontrolled pilot studies demonstrated promising results of endoscopic lung volume reduction using emphysematous lung sealant (ELS) in patients with advanced, upper lobe predominant emphysema. We aimed to evaluate the safety and efficacy of ELS in a randomised controlled setting.Patients were randomised to ELS plus medical treatment or medical treatment alone. Despite early termination for business reasons and inability to assess the primary 12-month end-point, 95 out of 300 patients were successfully randomised, providing sufficient data for 3- and 6-month analysis.57 patients (34 treatment and 23 control) had efficacy results at 3 months; 34 (21 treatment and 13 control) at 6 months. In the treatment group, 3-month lung function, dyspnoea, and quality of life improved significantly from baseline when compared to control. Improvements persisted at 6 months with >50% of treated patients experiencing clinically important improvements, including some whose lung function improved by >100%. 44% of treated patients experienced adverse events requiring hospitalisation (2.5-fold more than control, p=0.01), with two deaths in the treated cohort. Treatment responders tended to be those experiencing respiratory adverse events.Despite early termination, results show that minimally invasive ELS may be efficacious, yet significant risks (probably inflammatory) limit its current utility.
Trial registration: ClinicalTrials.gov NCT01449292.
Copyright ©ERS 2015.
Conflict of interest statement
Conflict of interest: Disclosures can be found alongside the online version of this article at
Figures
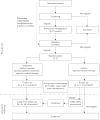
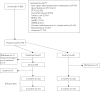
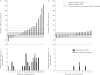

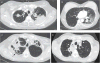
Comment in
-
When small is better: volume reduction in severe emphysema.Eur Respir J. 2015 Sep;46(3):593-5. doi: 10.1183/09031936.00065715. Eur Respir J. 2015. PMID: 26324687 No abstract available.
References
-
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. - PubMed
-
- Naunheim KS, Wood DE, Mohsenifar Z, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg. 2006;82:431–443. - PubMed
-
- Naunheim KS, Wood DE, Krasna MJ, et al. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg. 2006;131:43–53. - PubMed
-
- Akuthota P, Litmanovich D, Zutler M, et al. An evidence-based estimate on the size of the potential patient pool for lung volume reduction surgery. Ann Thorac Surg. 2012;94:205–211. - PubMed
-
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–1244. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
