Clinical and functional characterization of a novel mutation in lamin a/c gene in a multigenerational family with arrhythmogenic cardiac laminopathy
- PMID: 25837155
- PMCID: PMC4383583
- DOI: 10.1371/journal.pone.0121723
Clinical and functional characterization of a novel mutation in lamin a/c gene in a multigenerational family with arrhythmogenic cardiac laminopathy
Abstract
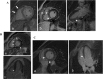
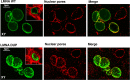
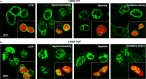
Mutations in the lamin A/C gene (LMNA) were associated with dilated cardiomyopathy (DCM) and, recently, were related to severe forms of arrhythmogenic right ventricular cardiomyopathy (ARVC). Both genetic and phenotypic overlap between DCM and ARVC was observed; molecular pathomechanisms leading to the cardiac phenotypes caused by LMNA mutations are not yet fully elucidated. This study involved a large Italian family, spanning 4 generations, with arrhythmogenic cardiomyopathy of different phenotypes, including ARVC, DCM, system conduction defects, ventricular arrhythmias, and sudden cardiac death. Mutation screening of LMNA and ARVC-related genes PKP2, DSP, DSG2, DSC2, JUP, and CTNNA3 was performed. We identified a novel heterozygous mutation (c.418_438dup) in LMNA gene exon 2, occurring in a highly conserved protein domain across several species. This newly identified variant was not found in 250 ethnically-matched control subjects. Genotype-phenotype correlation studies suggested a co-segregation of the LMNA mutation with the disease phenotype and an incomplete and age-related penetrance. Based on clinical, pedigree, and molecular genetic data, this mutation was considered likely disease-causing. To clarify its potential pathophysiologic impact, functional characterization of this LMNA mutant was performed in cultured cardiomyocytes expressing EGFP-tagged wild-type and mutated LMNA constructs, and indicated an increased nuclear envelope fragility, leading to stress-induced apoptosis as the main pathogenetic mechanism. This study further expands the role of the LMNA gene in the pathogenesis of cardiac laminopathies, suggesting that LMNA should be included in mutation screening of patients with suspected arrhythmogenic cardiomyopathy, particularly when they have ECG evidence for conduction defects. The combination of clinical, genetic, and functional data contribute insights into the pathogenesis of this form of life-threatening arrhythmogenic cardiac laminopathy.
Conflict of interest statement
Figures



References
-
- Taylor MR, Fain PR, Sinagra G, Robinson ML, Robertson AD, Carniel E, et al. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J Am Coll Cardiol. 2003;41: 771–780. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

